Contribution of GABAergic interneurons to the development of spontaneous activity patterns in cultured neocortical networks
- PMID: 20617185
- PMCID: PMC2896208
- DOI: 10.3389/fncel.2010.00015
Contribution of GABAergic interneurons to the development of spontaneous activity patterns in cultured neocortical networks
Abstract
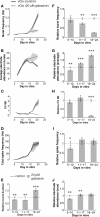
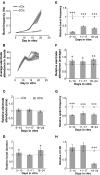
Periodic synchronized events are a hallmark feature of developing neuronal networks and are assumed to be crucial for the maturation of the neuronal circuitry. In the developing neocortex, the early network oscillations coincide with an excitatory action of the neurotransmitter gamma-aminobutyric acid (GABA). A relationship between the emerging inhibitory action of GABA and the gradual disappearance of early synchronized network activity has been previously suggested. Therefore we investigate the interplay between the action of GABA and spontaneous activity in cultured networks of the lateral or dorsal embryonic rat neocortex, which show considerable difference in the content of GABAergic neurons. Here we present the results of long-term monitoring of spontaneous electrical activity of cultured networks growing on microelectrode arrays and the time course of changes in GABA action using calcium imaging. All cultures studied displayed stereotyped synchronized burst events at the end of the first week in vitro. As the GABA(A) depolarizing action decreases, naturally or after bumetanide treatment, network activity in lateral cortex cultures changed from stereotypic bursting to more clustered and asynchronous activity patterns. Dorsal cortex cultures and cultures lacking GABA(A)-receptor mediated synaptic transmission, retained an immature synchronous firing pattern, but developed prominent intraburst oscillations ( approximately 3-10 Hz). Large, mostly parvalbumin positive, GABAergic neurons dominate the GABAergic population in lateral cortex cultures. These large interneurons were virtually absent in dorsal cortex cultures. Based on these results, we suggest that the richly interconnected large GABAergic neurons contribute to desynchronize and temporally differentiate the spontaneous activity of cultured cortical networks.
Keywords: MEA; cell culture; cerebral cortex; gamma-aminobutyric acid; neocortex; network activity; oscillations; rat.
Figures

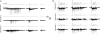


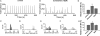





References
LinkOut - more resources
Full Text Sources
Research Materials

