Development of a safe and convenient neutralization assay for rapid screening of influenza HA-specific neutralizing monoclonal antibodies
- PMID: 20617558
- PMCID: PMC7092825
- DOI: 10.1016/j.bbrc.2010.05.161
Development of a safe and convenient neutralization assay for rapid screening of influenza HA-specific neutralizing monoclonal antibodies
Abstract
The worldwide outbreak of the swine-origin 2009 H1N1 influenza A virus (IAV) and an increasing number of influenza cases caused by a highly pathogenic avian influenza (HPAI) H5N1 have accelerated the need to develop vaccines and antiviral agents against IAVs. Among various antivirals, neutralizing monoclonal antibodies (mAbs) are considered important passive therapeutics having an immediate effect against viral pathogens. Here we report a pseudovirus neutralization assay for rapid screening of neutralizing mAbs targeting hemagglutinin (HA) of H5N1 and H1N1 IAV. In this study, we generated six pseudoviruses with an HIV-1 backbone, respectively, expressing HA of four clades of H5N1 IAV and the 2009 epidemic H1N1 IAV. The resulting pseudoviruses were able to infect a variety of human and non-human cells, with 293T cells from human kidney as the most susceptible target cells. Using the established pseudovirus neutralization assay, we showed that three of ten selected mAbs specific to HA could potently neutralize infection of a pseudovirus bearing HA from the homologous IAV A/VietNam/1194/2004(H5N1) strain. This was highly consistent with the result of a microneutralization assay testing the same strain of a live IAV. Since the pseudovirus neutralization assay does not involve an infectious virus and can be performed without the requirement of a biosafety-3 laboratory, it may be applied for safe and rapid screening of neutralizing mAbs and antiviral agents targeting HA of IAVs.
Figures

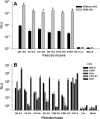

References
-
- Smith G.J., Vijaykrishna D., Bahl J. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
-
- Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

