Characteristics of hepatocellular carcinoma in a murine model of alpha-1-antitrypsin deficiency
- PMID: 20618460
- PMCID: PMC2928671
- DOI: 10.1111/j.1872-034X.2010.00663.x
Characteristics of hepatocellular carcinoma in a murine model of alpha-1-antitrypsin deficiency
Abstract
Aim: Individuals with homozygous (ZZ) alpha-1-antitrypsin (alpha1AT) deficiency are at an increased risk for liver damage, cirrhosis and hepatocellular carcinoma (HCC). The transgenic PiZ mouse, expressing the human alpha1AT mutant Z gene, is a valuable model for this disease. We studied PiZ mice in order to identify and characterize mechanisms involved in the development of HCC.
Methods: Tumor incidence and histology were studied, gene expression levels were surveyed with microarrays, RNA quantified with quantitative real time polymerase chain reaction and protein levels determined with immunoblots and immunohistochemistry.
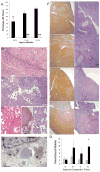
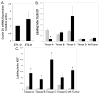
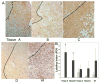
Results: By 16-19 months of age, approximately 69% of the PiZ mice had developed tumors. HCC was present with no evidence of benign adenomas as pre-cancerous lesions. Tumors showed abnormal mitochondria, variable levels of steatosis, globular inclusions of alpha1AT mutant Z protein and metastases. PiZ mice that subsequently developed liver tumors had higher serum levels of alpha1AT mutant Z protein than those that did not develop tumors. Cyclin D1, a cell cycle protein, was upregulated in PiZ livers without tumors compared to Wt. cFOS, a component of AP-1 that may be involved in transforming cells and MCAM, an adhesion molecule likely involved in tumorigenesis and metastases, were elevated in tumors compared with livers without tumors.
Conclusion: In the PiZ model, many of the histological characteristics of HCC recapitulated features seen in human HCC, whether from individuals with homozygous ZZ liver disease or from unrelated causes in individuals that were not homozygous ZZ. The accumulation of mutant Z protein altered the regulation of several genes driving proliferation and tumorigenesis.
Figures
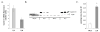




References
-
- Huber R, Carrell RW. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–66. - PubMed
-
- Teckman JH, Qu D, Perlmutter DH. Molecular pathogenesis of liver disease in alpha1-antitrypsin deficiency. Hepatology. 1996;24:1504–16. - PubMed
-
- Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology. 2005;42:514–21. - PubMed
-
- Propst T, Propst A, Dietze O, Judmaier G, Braunsteiner H, Vogel W. Prevalence of hepatocellular carcinoma in alpha-1-antitrypsin deficiency. J Hepatol. 1994;21:1006–11. - PubMed
-
- Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res. 2006;60:233–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

