Efficacy and safety of mitomycin C as an agent to treat corneal scarring in horses using an in vitro model
- PMID: 20618797
- PMCID: PMC2904635
- DOI: 10.1111/j.1463-5224.2010.00782.x
Efficacy and safety of mitomycin C as an agent to treat corneal scarring in horses using an in vitro model
Abstract
Objective: Mitomycin C (MMC) is used clinically to treat corneal scarring in human patients. We investigated the safety and efficacy of MMC to treat corneal scarring in horses by examining its effects at the early and late stages of disease using an in vitro model.
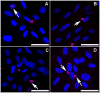
Procedure: An in vitro model of equine corneal fibroblast (ECF) developed was used. The ECF or myofibroblast cultures were produced by growing primary ECF in the presence or absence of transforming growth factor beta-1 (TGFbeta1) under serum-free conditions. The MMC dose for the equine cornea was defined with dose-dependent trypan blue exclusion and (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays after applying MMC to the cultures once for 2 min. The efficacy of MMC to control corneal scarring in horses was determined by measuring mRNA and protein expression of corneal scarring markers (alpha-smooth muscle actin and F-actin) with western blotting, immunocytochemistry and/or quantitative real-time polymerase chain reactions.
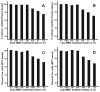
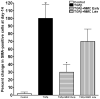
Results: A single 2-min treatment of 0.02% or less MMC did not alter ECF phenotype, viability, or cellular proliferation whereas 0.05% or higher MMC doses showed mild-to-moderate cellular toxicity. The TGFbeta1 at 1 ng/mL showed significant myofibroblast formation in ECF under serum-free conditions. A single 2-min, 0.02% MMC treatment 24 h (early) after TGFbeta1 stimulation significantly reduced conversion of ECF to myofibroblasts, however, a single 0.02% MMC treatment 11 days after TGFbeta1 stimulation showed moderate myofibroblast inhibition.
Conclusions: That MMC safely and effectively reduced scarring in ECF by reducing the degree of transdifferentiation of corneal fibroblasts to myofibroblasts in vitro. Further clinical in vivo investigations are warranted using MMC in horses.
Figures





References
-
- Haber M, Cao Z, Panjwani N, et al. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Veterinary Ophthalmology. 2003;6:211–217. - PubMed
-
- Michau TM, Schwabenton B, Davidson MG, et al. Superficial, nonhealing corneal ulcers in horses: 23 cases (1989–2003) Veterinary Ophthalmology. 2003;6:291–297. - PubMed
-
- Nasisse MP, Nelms S. Equine ulcerative keratitis. Vet Clinics North America Equine Practice. 1992;8:537–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

