Low levels of transforming growth factor-beta (TGF-beta) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis
- PMID: 20619070
- PMCID: PMC3004161
- DOI: 10.1017/S0031182010000922
Low levels of transforming growth factor-beta (TGF-beta) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis
Abstract
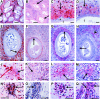
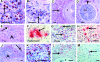
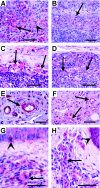
Th2-biased inflammation with eosinophilia and IgE production is a hallmark of helminth infections. It is pronounced in hyperreactive onchocerciasis patients ('sowda' or 'local form'), who efficiently kill microfilariae resulting in severe dermatitis and lymphadenitis. In contrast, hyporeactive patients ('generalised form') tolerate high microfilarial loads. This is thought to be mediated by regulatory CD4+ T cells and macrophages producing suppressive cytokines such as IL-10 and transforming growth factor-beta (TGF-β). We investigated whether hyperreactivity was reflected by lower local TGF-β production, analysing stable latent TGF-β1 expression in onchocercomas, lymph nodes and skin from hyperreactive and hyporeactive patients by immunohistochemistry. TGF-β expression was compared with that of IgE, IgG1, IgG4, and the antigen-presenting, CD4+ T cell-inducing MHC class II molecule HLA-DR. TGF-β was weakly and less frequently expressed by various cell types in onchocercomas, skin and lymph nodes from hyperreactive compared to hyporeactive patients. This applied to reactions around living and dead adult worms as well as dead microfilariae. Antigen-presenting cells strongly expressed HLA-DR in both forms, but their numbers were reduced in hyperreactive nodules. Plasma cells produced more IgE and IgG1, but less of the anti-inflammatory antibody IgG4 in hyperreactive onchocercomas. In conclusion, hyperreactivity is linked with reduced local expression of TGF-β, HLA-DR and IgG4, which might contribute to the insufficient down-regulation of inflammation via TGF-β- and HLA-DR-induced regulatory lymphocytes.
Figures
References
-
- Aalberse R. C., Stapel S. O., Schuurman J., Rispens T.. Immunoglobulin G4: an odd antibody. Clinical and Experimental Allergy. 2009;39:469–477. - PubMed
-
- Albiez E. J. Büttner D. W. Duke B. O. 1988aDiagnosis and extirpation of nodules in human onchocerciasis Tropical Medicine and Parasitology 39331–346. - PubMed
-
- Albiez E. J., Gallin M., Erttmann K. D., Racz P., Büttner D. W.. Characteristics of chronic severe onchodermatitis in Liberia. Journal of the Liberian Medical and Dental Association. 1985;15:120–125.
-
- Albiez E. J. Walter G. Kaiser A. Ranque P. Newland H. S. White A. T. Greene B. M. Taylor H. R. Büttner D. W. 1988bHistological examination of onchocercomata after therapy with ivermectin Tropical Medicine and Parasitology 3993–99. - PubMed
-
- Battaglia M., Roncarolo M. G.. The fate of human Treg cells. Immunity. 2009;30:763–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

