Tumorigenic and adhesive properties of heparanase
- PMID: 20619346
- PMCID: PMC2941534
- DOI: 10.1016/j.semcancer.2010.06.005
Tumorigenic and adhesive properties of heparanase
Abstract
Heparanase is an endo-β-glucuronidase that cleaves heparan sulfate side chains presumably at sites of low sulfation, activity that is strongly implicated with cell invasion associated with cancer metastasis, a consequence of structural modification that loosens the extracellular matrix barrier. In addition, heparanase exerts pro-adhesive properties, mediated by clustering of membrane heparan sulfate proteoglycans (i.e., syndecans) and activation of signaling molecules such as Akt, Src, EGFR, and Rac in a heparan sulfate-dependent and -independent manner. Activation of signaling cascades by enzymatically inactive heparanase and by a peptide corresponding to its substrate binding domain not only increases cell adhesion but also facilitates cancer cell growth. This notion is supported by preclinical and clinical settings, encouraging the development of anti-heparanase therapeutics. Here, we summarize recent progress in heparanase research emphasizing the molecular mechanisms that govern its pro-tumorigenic and pro-adhesive properties. Pro-adhesive properties of the heparanase homolog, heparanase 2 (Hpa2), are also discussed. Enzymatic activity-independent function of proteases (i.e., matrix metalloproteinases) is discussed in the context of cell adhesion and tumor progression. Collectively, these examples suggest that enzyme function exceeds beyond the enzymatic aspect, thus significantly expanding the scope of the functional proteome. Cross-talk with matrix metalloproteinases and the role of heparanase in pathological settings other than cancer are also described.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
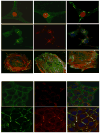
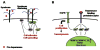
References
-
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. - PubMed
-
- Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–9. - PubMed
-
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–75. - PubMed
-
- Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

