Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults
- PMID: 20619382
- PMCID: PMC2923449
- DOI: 10.1016/j.vaccine.2010.06.084
Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults
Abstract
Background: Hepatitis C virus (HCV) causes chronic liver disease that often leads to cirrhosis and hepatocellular carcinoma. In animal studies, chimpanzees were protected against chronic infection following experimental challenge with either homologous or heterologous HCV genotype 1a strains which predominate in the USA and Canada. We describe the first in humans clinical trial of this prophylactic HCV vaccine.
Methods: HCV E1E2 adjuvanted with MF59C.1 (an oil-in-water emulsion) was given at 3 different dosages on day 0 and weeks 4, 24 and 48 in a phase 1, placebo-controlled, dose escalation trial to healthy HCV-negative adults.
Results: There was no significant difference in the proportion of subjects reporting adverse events across the groups. Following vaccination subjects developed antibodies detectable by ELISA, CD81 neutralization and VSV/HCV pseudotype neutralization. There were no significant differences between vaccine groups in the number of responders and geometric mean titers for each of the three assays. All subjects developed lymphocyte proliferation responses to E1E2 and an inverse response to increasing amounts of antigen was noted.
Conclusions: The vaccine was safe and generally well-tolerated at each of the 3 dosage levels and induced antibody and lymphoproliferative responses. A larger study to further evaluate safety and immunogenicity is warranted.
Trial registration: ClinicalTrials.gov NCT00500747.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures


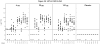
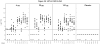
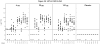
References
-
- Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. N Engl J Med. 1992;327(27):1906–11. - PubMed
-
- Alter MJ, Margolis HS, Krawcynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1992;327(27):1899–905. - PubMed
-
- Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–6. - PubMed
-
- Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterol. 1997;112(2):463–72. - PubMed
-
- Araya V, Rakela J, Wright T. Hepatitis C after orthotopic liver transplantation. Gastroenterol. 1997;112(2):575–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

