A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance
- PMID: 20619447
- PMCID: PMC3359143
- DOI: 10.1016/j.cell.2010.06.006
A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance
Abstract
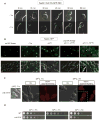
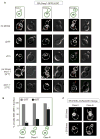
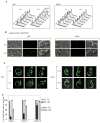
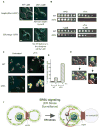
The endoplasmic reticulum (ER) plays an essential role in the production of lipids and secretory proteins. Because the ER cannot be generated de novo, it must be faithfully transmitted or divided at each cell division. Little is known of how cells monitor the functionality of the ER during the cell cycle or how this regulates inheritance. We report here that ER stress in S. cerevisiae activates the MAP kinase Slt2 in a new ER stress surveillance (ERSU) pathway, independent of the unfolded protein response. Upon ER stress, ERSU alters the septin complex to delay ER inheritance and cytokinesis. In the absence of Slt2 kinase, the stressed ER is transmitted to the daughter cell, causing the death of both mother and daughter cells. Furthermore, Slt2 is activated via the cell surface receptor Wsc1 by a previously undescribed mechanism. We conclude that the ERSU pathway ensures inheritance of a functional ER.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G, III, Patanwala I, Ng H-l, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: Supramolecular organization of heterooligomers and the mechanism of filament assembly. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8274–8279. - PMC - PubMed
-
- Bicknell AA, Niwa M. Regulating Endoplasmic Reticulum Function through the Unfolded Protein Response. Handbook of Cell Signaling. 2009:2511–2525.
-
- Bukau B, Weissman J, Horwich A. Molecular Chaperones and Protein Quality Control. Cell. 2006;123:443–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

