Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies
- PMID: 20619634
- PMCID: PMC3552301
- DOI: 10.1016/j.ejca.2010.06.002
Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies
Abstract
Introduction: Endometrial cancer patients with high grade tumours, deep myometrial invasion or advanced stage disease have a poor prognosis. Randomised studies have demonstrated the prevention of loco-regional relapses with radiotherapy (RT) with no effect on overall survival (OS). The possible additive effect of chemotherapy (CT) remains unclear. Two randomised clinical trials (NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III) were undertaken to clarify if sequential combination of chemotherapy and radiotherapy improves progression-free survival (PFS) in high-risk endometrial cancer. The two studies were pooled.
Methods: Patients (n=540; 534 evaluable) with operated endometrial cancer International Federation of Obstetrics and Gynaecology (FIGO) stage I-III with no residual tumour and prognostic factors implying high-risk were randomly allocated to adjuvant radiotherapy with or without sequential chemotherapy.
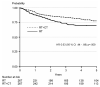
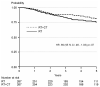
Results: In the NSGO/EORTC study, the combined modality treatment was associated with 36% reduction in the risk for relapse or death (hazard ratio (HR) 0.64, 95%confidence interval (CI) 0.41-0.99; P=0.04); two-sided tests were used. The result from the Gynaecologic Oncology group at the Mario Negri Institute (MaNGO)-study pointed in the same direction (HR 0.61), but was not significant. In the combined analysis, the estimate of risk for relapse or death was similar but with narrower confidence limits (HR 0.63, CI 0.44-0.89; P=0.009). Neither study showed significant differences in the overall survival. In the combined analysis, overall survival approached statistical significance (HR 0.69, CI 0.46-1.03; P=0.07) and cancer-specific survival (CSS) was significant (HR 0.55, CI 0.35-0.88; P=0.01).
Conclusion: Addition of adjuvant chemotherapy to radiation improves progression-free survival in operated endometrial cancer patients with no residual tumour and a high-risk profile. A remaining question for future studies is if addition of radiotherapy to chemotherapy improves the results.
Trial registration: ClinicalTrials.gov NCT00005583.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. - PubMed
-
- Aalders JG, Abeler VM, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma. Obstet Gynecol. 1980;56:419–26. - PubMed
-
- Creutzberg CL, van Putten WLJ, Koper PCM, Lybeert MLM, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. 2000;355:1404–11. - PubMed
-
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. - PubMed

