Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin
- PMID: 20620120
- PMCID: PMC2930122
- DOI: 10.1016/j.jpain.2010.05.007
Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin
Abstract
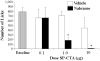
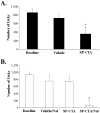
Individuals with chronic craniofacial pain experience symptoms that are consistent with central sensitization. In fact, central sensitization may constitute the major disease process in these conditions, particularly if the original injury has healed or the condition is idiopathic. To understand central sensitization we have developed a conjugate of substance P and cholera toxin (SP-CTA). SP-CTA is selectively taken up by cells that express neurokinin receptors. Twenty-four hours following intracisternal administration of SP-CTA, wild-type rats and mice demonstrated signs of persistent background nociception, but when tested for facial cold sensitivity, they did not differ from controls. However, treating the SP-CTA-injected animals with naloxone exposed cold hypersensitivity in the face. Mu-opioid receptor knockout mice treated with SP-CTA demonstrated hypersensitivity without naloxone treatment. These findings suggest that central sensitization leads to activation of an endogenous opioid system. The data also demonstrate that the intracisternal administration of SP-CTA in rodents is a useful model for studying central sensitization as a disease process without having to induce a peripheral injury.
Perspective: Central sensitization is a concern in many craniofacial pain conditions. In this project, we utilize a conjugate of substance P and the catalytic subunit of cholera toxin to induce central sensitization in the nucleus caudalis of rodents. The data indicate that the injected animals become hypersensitive in the face.
Copyright 2010 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Sleep-inducing effect of substance P-cholera toxin A subunit in mice.Neurosci Lett. 2017 Oct 17;659:44-47. doi: 10.1016/j.neulet.2017.08.066. Epub 2017 Sep 1. Neurosci Lett. 2017. PMID: 28866052 Free PMC article.
-
Anti-nociceptive effect of a conjugate of substance P and light chain of botulinum neurotoxin type A.Pain. 2013 Nov;154(11):2547-2553. doi: 10.1016/j.pain.2013.07.041. Epub 2013 Aug 8. Pain. 2013. PMID: 23933181 Free PMC article.
-
Sensitization of spinal cord nociceptive neurons with a conjugate of substance P and cholera toxin.BMC Neurosci. 2007 May 10;8:30. doi: 10.1186/1471-2202-8-30. BMC Neurosci. 2007. PMID: 17493276 Free PMC article.
-
Coactivation of μ- and κ-Opioid Receptors May Mediate the Protective Effect of Testosterone on the Development of Temporomandibular Joint Nociception in Male Rats.J Oral Facial Pain Headache. 2016 Winter;30(1):61-7. doi: 10.11607/ofph.1298. J Oral Facial Pain Headache. 2016. PMID: 26817034
-
Central serotonin 3 receptors play an important role in the modulation of nociceptive neural activity of trigeminal subnucleus caudalis and nocifensive orofacial behavior in rats with persistent temporomandibular joint inflammation.Neuroscience. 2005;135(2):569-81. doi: 10.1016/j.neuroscience.2005.06.032. Neuroscience. 2005. PMID: 16112478
Cited by
-
Pharmacological Characterization of Orofacial Nociception in Female Rats Following Nitroglycerin Administration.Front Pharmacol. 2020 Dec 3;11:527495. doi: 10.3389/fphar.2020.527495. eCollection 2020. Front Pharmacol. 2020. PMID: 33343340 Free PMC article.
-
Sleep-inducing effect of substance P-cholera toxin A subunit in mice.Neurosci Lett. 2017 Oct 17;659:44-47. doi: 10.1016/j.neulet.2017.08.066. Epub 2017 Sep 1. Neurosci Lett. 2017. PMID: 28866052 Free PMC article.
-
Characterization of bilateral trigeminal constriction injury using an operant facial pain assay.Neuroscience. 2012 Nov 8;224:294-306. doi: 10.1016/j.neuroscience.2012.08.015. Epub 2012 Aug 19. Neuroscience. 2012. PMID: 22909425 Free PMC article.
-
Paroxetine engenders analgesic effects through inhibition of p38 phosphorylation in a rat migraine model.Neural Regen Res. 2012 May 5;7(13):1006-12. doi: 10.3969/j.issn.1673-5374.2012.13.007. Neural Regen Res. 2012. PMID: 25722689 Free PMC article.
-
Peripheral and central substance P expression in rat CFA-induced TMJ synovitis pain.Mol Pain. 2019 Jan-Dec;15:1744806919866340. doi: 10.1177/1744806919866340. Mol Pain. 2019. PMID: 31322474 Free PMC article.
References
-
- Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. - PubMed
-
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. - PubMed
-
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123 (Pt 8):1703–1709. - PubMed
-
- Caudle RM, Mannes AJ, Benoliel R, Eliav E, Iadarola MJ. Intrathecally administered cholera toxin blocks allodynia and hyperalgesia in persistent pain models. Journal of Pain. 2001;2:118–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

