Macrophages, dendritic cells, and kidney ischemia-reperfusion injury
- PMID: 20620671
- PMCID: PMC2904394
- DOI: 10.1016/j.semnephrol.2010.03.005
Macrophages, dendritic cells, and kidney ischemia-reperfusion injury
Abstract
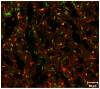
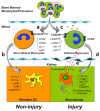
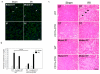
Dendritic cells and macrophages are critical early initiators of innate immunity in the kidney and orchestrate inflammation subsequent to ischemia-reperfusion injury. They are the most abundant leukocytes present in the kidney, and they represent a heterogeneous population of cells that are capable of inducing sterile inflammation after reperfusion directly through the production of proinflammatory cytokines and other soluble inflammatory mediators or indirectly through activation of effector T lymphocytes and natural killer T cells. In addition, recent studies have indicated that kidney and immune cell micro-RNAs control gene expression and have the ability to regulate the initial inflammatory response to injury. Although dendritic cells and macrophages contribute to both innate and adaptive immunity and to injury and repair, this review focuses on the initial innate response to kidney ischemia-reperfusion injury.
Figures

References
-
- Kaissling B, Le Hir M. Characterization and distribution of interstitial cell types in the renal cortex of rats. Kidney Int. 1994;45(3):709–20. - PubMed
-
- Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference? Kidney Int. 2008;74(1):5–7. - PubMed
-
- Li L, Okusa MD. Blocking the Immune respone in ischemic acute kidney injury: the role of adenosine 2A agonists. Nature Clinical Practice Nephrology. 2006;2:432–44. - PubMed
-
- Mason J, Torhorst J, Welsch J. Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int. 1984;26:283–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

