Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival
- PMID: 20620738
- PMCID: PMC2962668
- DOI: 10.1016/j.jacc.2010.04.016
Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival
Abstract
Objectives: The purpose of this study was to determine patient compliance and effectiveness of antiarrhythmic treatment by the wearable cardioverter-defibrillator (WCD).
Background: Effectiveness of the WCD for prevention of sudden death is dependent on event type, patient compliance, and appropriate management of ventricular tachycardia/ventricular fibrillation (VT/VF).
Methods: Compliance and events were recorded in a nationwide registry of post-market release WCDs. Survival, using the Social Security Death Index, was compared with survival in implantable cardioverter-defibrillator (ICD) patients.
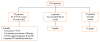
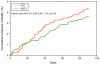
Results: Of 3,569 patients wearing the WCD (age 59.3+/-14.7 years, duration 52.6+/-69.9 days), daily use was 19.9+/-4.7 h (>90% of the day) in 52% of patients. More days of use correlated with higher daily use (p<0.001). Eighty sustained VT/VF events occurred in 59 patients (1.7%). First-shock success was 76 of 76 (100%) for unconscious VT/VF and 79 of 80 (99%) for all VT/VF. Eight patients died after successful conversion of unconscious VT/VF (89.5% survival of VT/VF events). Asystole occurred in 23 (17 died), pulseless electrical activity in 2, and respiratory arrest in 1 (3 died), representing 24.5% of sudden cardiac arrests. During WCD use, 3,541 of 3,569 patients (99.2%) survived overall. Survival occurred in 72 of 80 (90%) VT/VF events and 78 of 106 (73.6%) for all events. Long-term mortality was not significantly different from first ICD implant patients but highest among patients with traditional ICD indications.
Conclusions: Compliance was satisfactory with 90% wear time in >50% of patients and low sudden death mortality during use. Survival was comparable to that of ICD patients. However, asystole was an important cause of mortality in sudden cardiac arrest events.
Copyright (c) 2010 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures







Comment in
-
The wearable cardioverter-defibrillator: lifesaving attire or "fashion faux pas?".J Am Coll Cardiol. 2010 Jul 13;56(3):204-5. doi: 10.1016/j.jacc.2010.04.015. J Am Coll Cardiol. 2010. PMID: 20620739 No abstract available.
-
Wearable cardioverter–defibrillators in the spotlight.Nat Rev Cardiol. 2010 Oct;7(10):541. doi: 10.1038/nrcardio.2010.132. Nat Rev Cardiol. 2010. PMID: 21080557 No abstract available.
References
-
- A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–83. - PubMed
-
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. - PubMed
-
- Bokhari F, Newman D, Greene M, Korley V, Mangat I, Dorian P. Long-term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven-year follow-up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS) Circulation. 2004;110:112–6. - PubMed
-
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

