An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing
- PMID: 20620873
- PMCID: PMC2907746
- DOI: 10.1016/j.neuron.2010.05.009
An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing
Abstract
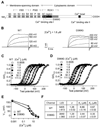
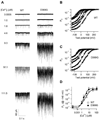
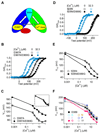
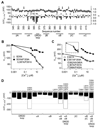
Ca(2+)-activated BK channels modulate neuronal activities, including spike frequency adaptation and synaptic transmission. Previous studies found that Ca(2+)-binding sites and the activation gate are spatially separated in the channel protein, but the mechanism by which Ca(2+) binding opens the gate over this distance remains unknown. By studying an Asp-to-Gly mutation (D434G) associated with human syndrome of generalized epilepsy and paroxysmal dyskinesia (GEPD), we show that a cytosolic motif immediately following the activation gate S6 helix, known as the AC region, mediates the allosteric coupling between Ca(2+) binding and channel opening. The GEPD mutation inside the AC region increases BK channel activity by enhancing this allosteric coupling. We found that Ca(2+) sensitivity is enhanced by increases in solution viscosity that reduce protein dynamics. The GEPD mutation alters such a response, suggesting that a less flexible AC region may be more effective in coupling Ca(2+) binding to channel opening.
Figures



Comment in
-
A less flexible BK channel opens more easily.Neuron. 2010 Jun 24;66(6):817-8. doi: 10.1016/j.neuron.2010.06.013. Neuron. 2010. PMID: 20620867 Free PMC article.
References
-
- Ansari A, Jones CM, Henry ER, Hofrichter J, Eaton WA. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992;256:1796–1798. - PubMed
-
- Ashcroft FM. Ion Channels and Disease: Channelopathies. San Diego, California, USA: Academic Press; 2000.
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Beece D, Eisenstein L, Frauenfelder H, Good D, Marden MC, Reinisch L, Reynolds AH, Sorensen LB, Yue KT. Solvent viscosity and protein dynamics. Biochemistry. 1980;19:5147–5157. - PubMed
-
- Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

