Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways
- PMID: 20620941
- PMCID: PMC2911434
- DOI: 10.1016/j.immuni.2010.06.002
Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways
Abstract
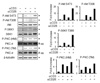
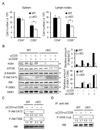
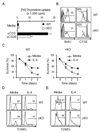
Many functions of the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) have been defined, but relatively little is known about the biology of an alternative mTOR complex, mTORC2. We showed that conditional deletion of rictor, an essential subunit of mTORC2, impaired differentiation into T helper 1 (Th1) and Th2 cells without diversion into FoxP3(+) status or substantial effect on Th17 cell differentiation. mTORC2 promoted phosphorylation of protein kinase B (PKB, or Akt) and PKC, Akt activity, and nuclear NF-kappaB transcription factors in response to T cell activation. Complementation with active Akt restored only T-bet transcription factor expression and Th1 cell differentiation, whereas activated PKC-theta reverted only GATA3 transcription factor and the Th2 cell defect of mTORC2 mutant cells. Collectively, the data uncover vital mTOR-PKC and mTOR-Akt connections in T cell differentiation and reveal distinct pathways by which mTORC2 regulates development of Th1 and Th2 cell subsets.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Comment in
-
T helper cell differentiation: understanding the needs of hierarchy.Immunity. 2010 Jun 25;32(6):727-9. doi: 10.1016/j.immuni.2010.06.008. Immunity. 2010. PMID: 20620936
References
-
- Andres P, Howland K, Nirula A, Kane L, Barron L, Dresnek D, Sadra A, Imboden J, Weiss A, Abbas AK. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat Immunol. 2004;5:435–442. - PubMed
-
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. - PubMed
-
- Blazar B, Taylor P, Panoskaltsis-Mortari A, Vallera D. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J Immunol. 1998;160:5355–5365. - PubMed
-
- Cannons JL, Yu L, Hill B, Mijares L, Dombroski D, Nichols KE, Antonellis A, Koretzky G, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. - PubMed
-
- Carriere A, Cargnello M, Julien L, Gao H, Bonneil E, Thibault P, Roux P. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

