Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue
- PMID: 20620944
- PMCID: PMC3742307
- DOI: 10.1016/j.immuni.2010.06.007
Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue
Abstract
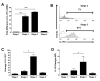
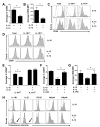
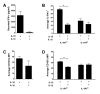
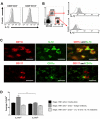
Among human natural killer (NK) cell intermediates in secondary lymphoid tissue (SLT), stage 3 CD34(-)CD117(+)CD161(+)CD94(-) immature NK (iNK) cells uniquely express aryl hydrocarbon receptor (AHR) and interleukin-22 (IL-22), supporting a role in mucosal immunity. The mechanisms controlling proliferation and differentiation of these cells are unknown. Here we demonstrate that the IL-1 receptor IL-1R1 was selectively expressed by a subpopulation of iNK cells that localized proximal to IL-1beta-producing conventional dendritic cells (cDCs) within SLT. IL-1R1(hi) iNK cells required continuous exposure to IL-1beta to retain AHR and IL-22 expression, and they proliferate in direct response to cDC-derived IL-15 and IL-1beta. In the absence of IL-1beta, a substantially greater fraction of IL-1R1(hi) iNK cells differentiated to stage 4 NK cells and acquired the ability to kill and secrete IFN-gamma. Thus, cDC-derived IL-1beta preserves and expands IL-1R1(hi)IL-22(+)AHR(+) iNK cells, potentially influencing human mucosal innate immunity during infection.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Comment in
-
An IL-1beta-dependent switch in innate mucosal immunity?Immunity. 2010 Jun 25;32(6):734-6. doi: 10.1016/j.immuni.2010.06.012. Immunity. 2010. PMID: 20620939
References
-
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. - PubMed
-
- Arend WP, Malyak M, Bigler CF, Smith MF, Jr., Janson RW. The biological role of naturally-occurring cytokine inhibitors. Br J Rheumatol. 1991;30(Suppl 2):49–52. - PubMed
-
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. - PubMed
-
- Carter DB, Deibel MR, Jr., Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

