GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries
- PMID: 20620994
- PMCID: PMC2913606
- DOI: 10.1016/j.cmet.2010.04.016
GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries
Abstract
The lipolytic processing of triglyceride-rich lipoproteins by lipoprotein lipase (LPL) is the central event in plasma lipid metabolism, providing lipids for storage in adipose tissue and fuel for vital organs such as the heart. LPL is synthesized and secreted by myocytes and adipocytes, but then finds its way into the lumen of capillaries, where it hydrolyzes lipoprotein triglycerides. The mechanism by which LPL reaches the lumen of capillaries has remained an unresolved problem of plasma lipid metabolism. Here, we show that GPIHBP1 is responsible for the transport of LPL into capillaries. In Gpihbp1-deficient mice, LPL is mislocalized to the interstitial spaces surrounding myocytes and adipocytes. Also, we show that GPIHBP1 is located at the basolateral surface of capillary endothelial cells and actively transports LPL across endothelial cells. Our experiments define the function of GPIHBP1 in triglyceride metabolism and provide a mechanism for the transport of LPL into capillaries.
Copyright 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
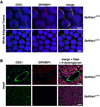

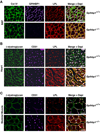
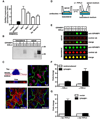
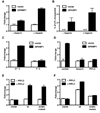

Comment in
-
GPIHBP1: lipoprotein lipase's ticket to ride.Cell Metab. 2010 Jul 7;12(1):1-2. doi: 10.1016/j.cmet.2010.06.005. Cell Metab. 2010. PMID: 20620987
References
-
- Anderson RG. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993;5:647–652. - PubMed
-
- Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. - PMC - PubMed
-
- Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009a;29:956–962. - PMC - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

