Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone
- PMID: 20621668
- PMCID: PMC3072757
- DOI: 10.1016/j.jmb.2010.05.067
Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone
Abstract
Human gamma-crystallins are long-lived, unusually stable proteins of the eye lens exhibiting duplicated, double Greek key domains. The lens also contains high concentrations of the small heat shock chaperone alpha-crystallin, which suppresses aggregation of model substrates in vitro. Mature-onset cataract is believed to represent an aggregated state of partially unfolded and covalently damaged crystallins. Nonetheless, the lack of cell or tissue culture for anucleate lens fibers and the insoluble state of cataract proteins have made it difficult to identify the conformation of the human gamma-crystallin substrate species recognized by human alpha-crystallin. The three major human lens monomeric gamma-crystallins, gammaD, gammaC, and gammaS, all refold in vitro in the absence of chaperones, on dilution from denaturant into buffer. However, off-pathway aggregation of the partially folded intermediates competes with productive refolding. Incubation with human alphaB-crystallin chaperone during refolding suppressed the aggregation pathways of the three human gamma-crystallin proteins. The chaperone did not dissociate or refold the aggregated chains under these conditions. The alphaB-crystallin oligomers formed long-lived stable complexes with their gammaD-crystallin substrates. Using alpha-crystallin chaperone variants lacking tryptophans, we obtained fluorescence spectra of the chaperone-substrate complex. Binding of substrate gamma-crystallins with two or three of the four buried tryptophans replaced by phenylalanines showed that the bound substrate remained in a partially folded state with neither domain native-like. These in vitro results provide support for protein unfolding/protein aggregation models for cataract, with alpha-crystallin suppressing aggregation of damaged or unfolded proteins through early adulthood but becoming saturated with advancing age.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures

 ) and unfolded (●) αB-Crys at 37°C. Protein concentration for far-UV CD and fluorescence emission measurements was 100 μg/ml.
) and unfolded (●) αB-Crys at 37°C. Protein concentration for far-UV CD and fluorescence emission measurements was 100 μg/ml.

 ) and 1 γD:5 αB mixture of native proteins (—) chromatograms are shown. B) 1 γC:5 αB suppression reaction (
) and 1 γD:5 αB mixture of native proteins (—) chromatograms are shown. B) 1 γC:5 αB suppression reaction ( ) and 1 γC:5 αB mixture of native proteins (—) chromatograms are shown. C) 1 γS:5 αB suppression reaction (
) and 1 γC:5 αB mixture of native proteins (—) chromatograms are shown. C) 1 γS:5 αB suppression reaction ( ) and 1 γS:5 αB mixture of native proteins (—) chromatograms are shown. The suppression reactions shown in Fig. 2 were applied to a Superose 6 size-exclusion column (for the native mixture samples’ preparation see Materials and Methods) Arrows correspond to elution volume of standards. Numbers represent standards’ molecular weights in kDa.
) and 1 γS:5 αB mixture of native proteins (—) chromatograms are shown. The suppression reactions shown in Fig. 2 were applied to a Superose 6 size-exclusion column (for the native mixture samples’ preparation see Materials and Methods) Arrows correspond to elution volume of standards. Numbers represent standards’ molecular weights in kDa.

 ) at 25°C. λEX was set to 300 nm. B) W9F/W60F αB-Crys suppressed the aggregation of γD-Crys under the same refolding and aggregation described previously (see Materials and Methods). Final γD-Crys concentration was 100 μg/ml and final GdnHCl concentration was 0.5 M at 37°C. C) A high molecular weight complex could be isolated from the 1γD:5 noTrp αB suppression reactions during SEC. The volume highlighted by the gray bar corresponds to the fraction collected for fluorescence measurements. D) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex (
) at 25°C. λEX was set to 300 nm. B) W9F/W60F αB-Crys suppressed the aggregation of γD-Crys under the same refolding and aggregation described previously (see Materials and Methods). Final γD-Crys concentration was 100 μg/ml and final GdnHCl concentration was 0.5 M at 37°C. C) A high molecular weight complex could be isolated from the 1γD:5 noTrp αB suppression reactions during SEC. The volume highlighted by the gray bar corresponds to the fraction collected for fluorescence measurements. D) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex ( ). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) WT γD-Crys (20 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.
). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) WT γD-Crys (20 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys.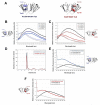
 ) or W42F/W68F (
) or W42F/W68F ( ) γD-Crys as substrates. The shaded area on the trace indicates the fraction used for fluorescence measurements. E) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex from 1 W130F/W156F γD: 5 noTrp αB suppression reaction (
) γD-Crys as substrates. The shaded area on the trace indicates the fraction used for fluorescence measurements. E) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex from 1 W130F/W156F γD: 5 noTrp αB suppression reaction ( ). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) W130F/W156F γD-Crys (50 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys. F) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex isolated from 1 W42F/W68F γD: 5 noTrp αB suppression reaction (
). For comparison purposes, the fluorescence emission spectrum of native (○) and fully unfolded (●) W130F/W156F γD-Crys (50 μg/ml) are shown. The λEX was set to 300 nm for all samples. Traces were normalized to the fluorescence intensity of the maximum λEM for unfolded γD-Crys. F) Relative fluorescence emission spectrum of the fraction containing the γD—αB complex isolated from 1 W42F/W68F γD: 5 noTrp αB suppression reaction ( ). The λEX was set to 300 nm for all samples and the protein concentration for the native (○) and fully unfolded (●) W42F/W68F γD-Crys protein samples was 50 μg/ml. Traces were normalized as described above.
). The λEX was set to 300 nm for all samples and the protein concentration for the native (○) and fully unfolded (●) W42F/W68F γD-Crys protein samples was 50 μg/ml. Traces were normalized as described above.
 ). B) Fluorescence emission spectra for W130only γD-Crys in complex with noTrp αB-Crys (
). B) Fluorescence emission spectra for W130only γD-Crys in complex with noTrp αB-Crys ( ). Spectra for their respective native (○) and unfolded (●) controls is also shown (protein concentration for W42only γD-Crys was 50 μg/ml and for W130only γD-Crys was 25 μg/ml). The λEX was 300 nm and the traces were normalized as described previously in Figure 6. Complexes were isolated from 1γD:5αB suppression reactions in which the final concentration of γD was 50 μg/ml.
). Spectra for their respective native (○) and unfolded (●) controls is also shown (protein concentration for W42only γD-Crys was 50 μg/ml and for W130only γD-Crys was 25 μg/ml). The λEX was 300 nm and the traces were normalized as described previously in Figure 6. Complexes were isolated from 1γD:5αB suppression reactions in which the final concentration of γD was 50 μg/ml.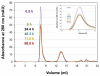


References
-
- Ponce A, Sorensen C, Takemoto L. Role of short-range protein interactions in lens opacifications. Mol Vis. 2006;12:879–84. - PubMed
-
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. - PubMed
-
- de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–26. - PubMed
-
- Robinson NE, Lampi KJ, Speir JP, Kruppa G, Easterling M, Robinson AB. Quantitative measurement of young human eye lens crystallins by direct injection Fourier transform ion cyclotron resonance mass spectrometry. Mol Vis. 2006;12:704–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

