Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors
- PMID: 20621681
- PMCID: PMC2933938
- DOI: 10.1053/j.gastro.2010.05.083
Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors
Abstract
Background & aims: Gastrointestinal stromal tumors (GIST) are related to interstitial cells of Cajal (ICC) and often contain activating stem cell factor receptor (Kit) or platelet-derived growth factor receptor alpha (Pdgfra) mutations. Kit/Pdgfra inhibitors such as imatinib mesylate have increased progression-free survival in metastatic GIST but are not curative. In mouse models we investigated whether Kit(low) ICC progenitors could represent an inherently Kit/Pdgfra inhibitor-resistant reservoir for GIST.
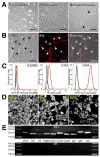
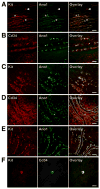
Methods: Isolated Kit(low)Cd44(+)Cd34(+) cells were characterized after serial cloning. The tumorigenic potential of spontaneously transformed cells was investigated in nude mice. The Kit(low)Cd44(+)Cd34(+) cells' responsiveness to Kit activation and blockade was studied by enumerating them in Kit(K641E) mice (a GIST model), in mice with defective Kit signaling, and pharmacologically.
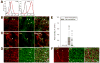
Results: Single isolated Kit(low)Cd44(+)Cd34(+) cells were clonogenic and capable of self-renewal and differentiation into ICC. In nude mice, spontaneously transformed cells formed malignant tumors expressing GIST markers. The Kit(low)Cd44(+)Cd34(+) cells were resistant to in vitro Kit blockade, including by imatinib, and occurred in normal numbers in mice with reduced Kit signaling. In Kit(K641E) mice, the mutant ICC stem cells were grossly hyperplastic but remained imatinib-resistant. In contrast, the cancer stem, cell-targeting drug salinomycin blocked the proliferation of Kit(low)Cd44(+)Cd34(+) cells and increased their sensitivity to imatinib.
Conclusions: Kit(low)Cd44(+)Cd34(+) progenitors are true stem cells for normal and hyperplastic ICC and give rise to GIST. Resistance to Kit/Pdgfra inhibitors is inherent in GIST and is caused by the native ICC stem cells' lack of dependence on Kit for survival, which is maintained after the acquisition of oncogenic Kit mutation. Cancer stem cell drugs may target these cells.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


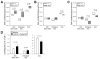

References
-
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. - PubMed
-
- Sanders KM, Ordog T, Koh SD, et al. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–338. - PubMed
-
- Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

