Therapeutic liver reconstitution with murine cells isolated long after death
- PMID: 20621682
- PMCID: PMC3786690
- DOI: 10.1053/j.gastro.2010.05.082
Therapeutic liver reconstitution with murine cells isolated long after death
Abstract
Background & aims: Due to the shortage of donor organs, many patients needing liver transplantation cannot receive one. For some liver diseases, hepatocyte transplantation could be a viable alternative, but donor cells currently are procured from the same sources as whole organs, and thus the supply is severely limited.
Methods: Here, we investigated the possibility of isolating viable hepatocytes for liver cell therapy from the plentiful source of morgue cadavers. To determine the utility of this approach, cells were isolated from the livers of non-heart-beating cadaveric mice long after death and transplanted into fumarylacetoacetate hydrolase-deficient mice, a model for the human metabolic liver disease hereditary tyrosinemia type I and a stringent in vivo model for hepatic cell transplantation.
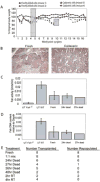
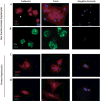
Results: Surprisingly, complete and therapeutic liver repopulation could be achieved with hepatocytes derived up to 27 hours post mortem.
Conclusions: Competitive repopulation experiments showed that cadaveric liver cells had a repopulation capacity similar to freshly isolated hepatocytes. Importantly, viable hepatocytes also could be isolated from cadaveric primate liver (monkey and human) efficiently. These data provide evidence that non-heart-beating donors could be a suitable source of hepatocytes for much longer time periods than previously thought possible.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest to disclose for all authors.
Figures




Comment in
-
Cadaveric hepatocytes repopulate diseased livers: life after death.Gastroenterology. 2010 Sep;139(3):729-31. doi: 10.1053/j.gastro.2010.07.013. Epub 2010 Jul 24. Gastroenterology. 2010. PMID: 20659459 No abstract available.
References
-
- Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–9. - PubMed
-
- Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–9. - PMC - PubMed
-
- Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, Pryde A, Filippi C, Currie IS, Forbes SJ, Ross JA, Newsome PN, Iredale JP. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–6. - PMC - PubMed
-
- Cantz T, Zuckerman DM, Burda MR, Dandri M, Goricke B, Thalhammer S, Heckl WM, Manns MP, Petersen J, Ott M. Quantitative gene expression analysis reveals transition of fetal liver progenitor cells to mature hepatocytes after transplantation in uPA/RAG-2 mice. Am J Pathol. 2003;162:37–45. - PMC - PubMed
-
- Sharma AD, Cantz T, Vogel A, Schambach A, Haridass D, Iken M, Bleidissel M, Manns MP, Scholer HR, Ott M. Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 2008;17:313–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

