Complement activation on platelets: implications for vascular inflammation and thrombosis
- PMID: 20621693
- PMCID: PMC2904326
- DOI: 10.1016/j.molimm.2010.05.009
Complement activation on platelets: implications for vascular inflammation and thrombosis
Abstract
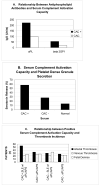
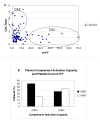
Platelets participate in a variety of responses of the blood to injury. An emerging body of evidence suggests that these cells express an intrinsic capacity to interact with and trigger both classical and alternative pathways of complement. This activity requires cell activation with biochemical agonists and/or shear stress, and is associated with the expression of P-selectin, gC1qR, and chondroitin sulfate. Platelet mediated complement activation measurably increases soluble inflammatory mediators (C3a and C5a). Platelets may also serve as targets of classical complement activation in autoimmune conditions such as antiphospholipid syndromes (APS) and immune thrombocytopenia purpura (ITP). Retrospective correlation with clinical data suggests that enhanced platelet associated complement activation correlates with increased arterial thrombotic events in patients with lupus erythematosus and APS, and evidence of enhanced platelet clearance from the circulation in patients with ITP. Taken together, these data support a role for platelet mediated complement activation in vascular inflammation and thrombosis.
Keywords: Platelets; antiphospholipid syndrome; complement; immune thrombocytopenia purpura; inflammation; systemic lupus erythematosus; thrombosis.
2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. - PubMed
-
- Choudhury A, Chung I, Blann AD, Lip GY. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest. 2007;131:809–815. - PubMed
-
- Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin Exp Immunol. 1992;10:445–460. - PubMed
-
- DeJong A, Siboh V, Robbins D. Antiphospholipid antibodies and platelets. Curr Rheum Rep. 2000;2:238–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

