Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins
- PMID: 20621699
- PMCID: PMC3075980
- DOI: 10.1053/j.gastro.2010.05.077
Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins
Abstract
Background & aims: Studies in patients and chimpanzees that spontaneously cleared hepatitis C virus (HCV) infections demonstrated that natural immunity to the virus is induced during primary infections and that this immunity can be cross protective. These discoveries led to optimism about prophylactic HCV vaccines, and several studies were performed in chimpanzees, although most included fewer than 6 animals. To draw meaningful conclusions about the efficacy of HCV vaccines in chimpanzees, we performed statistical analyses of data from previously published studies from different groups.
Methods: We performed a meta-analysis that compared parameters among naïve (n = 63), vaccinated (n = 53), and rechallenged (n = 36) animals, including peak RNA titer postchallenge, time points of peak RNA titer, duration of viremia, and proportion of persistent infections.
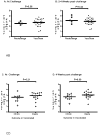
Results: Each vaccination study induced immune responses that were effective in rapidly controlling HCV replication. Levels of induced T-cell responses did not indicate vaccine success. There was no reduction in the rate of HCV persistence in vaccinated animals, compared with naïve animals, when nonstructural proteins were included in the vaccine. Vaccines that contained only structural proteins had clearance rates that were significantly higher than vaccines that contained nonstructural components (P = .015).
Conclusions: The inclusion of nonstructural proteins in HCV vaccines might be detrimental to protective immune responses, and/or structural proteins might activate T-cell responses that mediate viral clearance.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: The authors have no conflicts of interest to disclose.
Figures
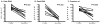
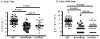
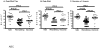
References
-
- Bartenschlager R, Frese M, Pietschmann T. Novel insights into hepatitis C virus replication and persistence. Adv Virus Res. 2004;63:71–180. - PubMed
-
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. - PubMed
-
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. - PubMed
-
- Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. - PubMed
-
- National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

