Analysis of site-specific glycosylation of renal and hepatic γ-glutamyl transpeptidase from normal human tissue
- PMID: 20622017
- PMCID: PMC2937983
- DOI: 10.1074/jbc.M110.145938
Analysis of site-specific glycosylation of renal and hepatic γ-glutamyl transpeptidase from normal human tissue
Abstract
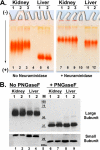
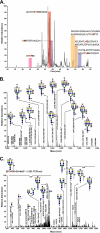
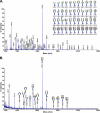
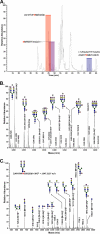
The cell surface glycoprotein γ-glutamyl transpeptidase (GGT) was isolated from healthy human kidney and liver to characterize its glycosylation in normal human tissue in vivo. GGT is expressed by a single cell type in the kidney. The spectrum of N-glycans released from kidney GGT constituted a subset of the N-glycans identified from renal membrane glycoproteins. Recent advances in mass spectrometry enabled us to identify the microheterogeneity and relative abundance of glycans on specific glycopeptides and revealed a broader spectrum of glycans than was observed among glycans enzymatically released from isolated GGT. A total of 36 glycan compositions, with 40 unique structures, were identified by site-specific glycan analysis. Up to 15 different glycans were observed at a single site, with site-specific variation in glycan composition. N-Glycans released from liver membrane glycoproteins included many glycans also identified in the kidney. However, analysis of hepatic GGT glycopeptides revealed 11 glycan compositions, with 12 unique structures, none of which were observed on kidney GGT. No variation in glycosylation was observed among multiple kidney and liver donors. Two glycosylation sites on renal GGT were modified exclusively by neutral glycans. In silico modeling of GGT predicts that these two glycans are located in clefts on the surface of the protein facing the cell membrane, and their synthesis may be subject to steric constraints. This is the first analysis at the level of individual glycopeptides of a human glycoprotein produced by two different tissues in vivo and provides novel insights into tissue-specific and site-specific glycosylation in normal human tissues.
Figures


Similar articles
-
Detection of distinct glycosylation patterns on human γ-glutamyl transpeptidase 1 using antibody-lectin sandwich array (ALSA) technology.BMC Biotechnol. 2014 Dec 6;14:101. doi: 10.1186/s12896-014-0101-0. BMC Biotechnol. 2014. PMID: 25479762 Free PMC article.
-
Autocatalytic cleavage of human gamma-glutamyl transpeptidase is highly dependent on N-glycosylation at asparagine 95.J Biol Chem. 2011 Aug 19;286(33):28876-28888. doi: 10.1074/jbc.M111.248823. Epub 2011 Jun 28. J Biol Chem. 2011. PMID: 21712391 Free PMC article.
-
Site-specific glycosylation analysis of the bovine lysosomal alpha-mannosidase.Glycobiology. 2006 May;16(5):440-61. doi: 10.1093/glycob/cwj081. Epub 2006 Jan 31. Glycobiology. 2006. PMID: 16449350
-
Methods in enzymology: O-glycosylation of proteins.Methods Enzymol. 2005;405:139-71. doi: 10.1016/S0076-6879(05)05007-X. Methods Enzymol. 2005. PMID: 16413314 Review.
-
Structural, functional, and clinical aspects of gamma-glutamyltransferase.CRC Crit Rev Clin Lab Sci. 1980;12(1):1-58. doi: 10.3109/10408368009108725. CRC Crit Rev Clin Lab Sci. 1980. PMID: 6104563 Review.
Cited by
-
Human GGT2 does not autocleave into a functional enzyme: A cautionary tale for interpretation of microarray data on redox signaling.Antioxid Redox Signal. 2013 Dec 1;19(16):1877-88. doi: 10.1089/ars.2012.4997. Epub 2013 Jun 28. Antioxid Redox Signal. 2013. PMID: 23682772 Free PMC article.
-
Detection of distinct glycosylation patterns on human γ-glutamyl transpeptidase 1 using antibody-lectin sandwich array (ALSA) technology.BMC Biotechnol. 2014 Dec 6;14:101. doi: 10.1186/s12896-014-0101-0. BMC Biotechnol. 2014. PMID: 25479762 Free PMC article.
-
Novel insights into eukaryotic γ-glutamyltranspeptidase 1 from the crystal structure of the glutamate-bound human enzyme.J Biol Chem. 2013 Nov 1;288(44):31902-13. doi: 10.1074/jbc.M113.498139. Epub 2013 Sep 18. J Biol Chem. 2013. PMID: 24047895 Free PMC article.
-
Human Angiotensin I-Converting Enzyme Produced by Different Cells: Classification of the SERS Spectra with Linear Discriminant Analysis.Biomedicines. 2022 Jun 12;10(6):1389. doi: 10.3390/biomedicines10061389. Biomedicines. 2022. PMID: 35740411 Free PMC article.
-
Increased risk of acute pancreatitis occurrence in smokers with rs5751901 polymorphisms in GGT1 gene.Int J Med Sci. 2020 Jan 14;17(2):242-254. doi: 10.7150/ijms.38657. eCollection 2020. Int J Med Sci. 2020. PMID: 32038108 Free PMC article.
References
-
- Helenius A., Aebi M. (2001) Science 291, 2364–2369 - PubMed
-
- Haltiwanger R. S., Lowe J. B. (2004) Annu. Rev. Biochem. 73, 491–537 - PubMed
-
- Haslam S. M., North S. J., Dell A. (2006) Curr. Opin. Struct. Biol. 16, 584–591 - PubMed
-
- Comelli E. M., Head S. R., Gilmartin T., Whisenant T., Haslam S. M., North S. J., Wong N. K., Kudo T., Narimatsu H., Esko J. D., Drickamer K., Dell A., Paulson J. C. (2006) Glycobiology 16, 117–131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

