Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children
- PMID: 20622026
- PMCID: PMC3001264
- DOI: 10.1164/rccm.201004-0643OC
Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children
Abstract
Rationale: Although airway abnormalities are common in patients with cystic fibrosis (CF), it is unknown whether they are all secondary to postnatal infection and inflammation, which characterize the disease.
Objectives: To learn whether loss of the cystic fibrosis transmembrane conductance regulator (CFTR) might affect major airways early in life, before the onset of inflammation and infection.
Methods: We studied newborn CFTR⁻(/)⁻ pig trachea, using computed tomography (CT) scans, pathology, and morphometry. We retrospectively analyzed trachea CT scans in young children with CF and also previously published data of infants with CF.
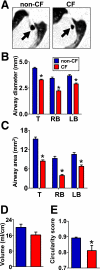
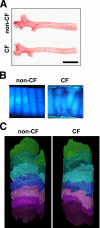
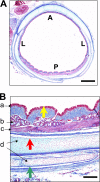
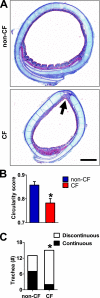
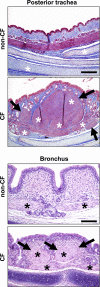
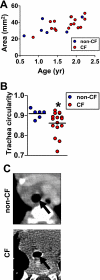
Measurements and main results: We discovered three abnormalities in the porcine CF trachea. First, the trachea and mainstem bronchi had a uniformly small caliber and cross-sections of trachea were less circular than in controls. Second, trachealis smooth muscle had an altered bundle orientation and increased transcripts in a smooth muscle gene set. Third, submucosal gland units occurred with similar frequency in the mucosa of CF and control airways, but CF submucosal glands were hypoplastic and had global reductions in tissue-specific transcripts. To learn whether any of these changes occurred in young patients with CF, we examined CT scans from children 2 years of age and younger, and found that CF tracheas were less circular in cross-section, but lacked differences in lumen area. However, analysis of previously published morphometric data showed reduced tracheal lumen area in neonates with CF.
Conclusions: Our findings in newborn CF pigs and young patients with CF suggest that airway changes begin during fetal life and may contribute to CF pathogenesis and clinical disease during postnatal life.
Figures
References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073. - PubMed
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 2001. pp. 5121–5189.
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001. - PubMed
-
- O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009;373:1891–1904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

