Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy
- PMID: 20622029
- PMCID: PMC3001266
- DOI: 10.1164/rccm.201002-0271OC
Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy
Abstract
Rationale: We identified a 6-year-old girl with pulmonary alveolar proteinosis (PAP), impaired granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor function, and increased GM-CSF.
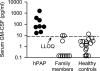
Objectives: Increased serum GM-CSF may be useful to identify individuals with PAP caused by GM-CSF receptor dysfunction.
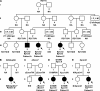
Methods: We screened 187 patients referred to us for measurement of GM-CSF autoantibodies to diagnose autoimmune PAP. Five were children with PAP and increased serum GM-CSF but without GM-CSF autoantibodies or any disease causing secondary PAP; all were studied with family members, subsequently identified patients, and controls.
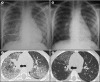
Measurement and main results: Eight children (seven female, one male) were identified with PAP caused by recessive CSF2RA mutations. Six presented with progressive dyspnea of insidious onset at 4.8 ± 1.6 years and two were asymptomatic at ages 5 and 8 years. Radiologic and histopathologic manifestations were similar to those of autoimmune PAP. Molecular analysis demonstrated that GM-CSF signaling was absent in six and severely reduced in two patients. The GM-CSF receptor β chain was detected in all patients, whereas the α chain was absent in six and abnormal in two, paralleling the GM-CSF signaling defects. Genetic analysis revealed multiple distinct CSF2RA abnormalities, including missense, duplication, frameshift, and nonsense mutations; exon and gene deletion; and cryptic alternative splicing. All symptomatic patients responded well to whole-lung lavage therapy.
Conclusions: CSF2RA mutations cause a genetic form of PAP presenting as insidious, progressive dyspnea in children that can be diagnosed by a combination of characteristic radiologic findings and blood tests and treated successfully by whole-lung lavage.
Figures





References
-
- Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol 2001;63:495–519. - PubMed
-
- Ikegami M, Ueda T, Hull W, Whitsett JA, Mulligan RC, Dranoff G, Jobe AH. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol 1996;270:L650–L658. - PubMed
-
- Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through pu.1. Immunity 2001;15:557–567. - PubMed
-
- Yoshida M, Ikegami M, Reed JA, Chroneos ZC, Whitsett JA. GM-CSF regulates surfactant protein-A and lipid catabolism by alveolar macrophages. Am J Physiol 2001;280:L379–L386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

