Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats
- PMID: 20622037
- PMCID: PMC3001261
- DOI: 10.1164/rccm.201001-0047OC
Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats
Abstract
Rationale: Hypoventilation is typically treated with positive pressure ventilation or, in extreme cases, by phrenic nerve stimulation. This preclinical study explores whether direct stimulation of central chemoreceptors could be used as an alternative method to stimulate breathing.
Objectives: To determine whether activation of the retrotrapezoid nucleus (RTN), which is located in the rostral ventrolateral medulla (RVLM), stimulates breathing with appropriate selectivity.
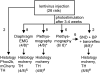
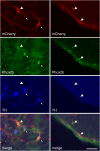
Methods: A lentivirus was used to induce expression of the photoactivatable cationic channel channelrhodopsin-2 (ChR2) by RVLM Phox2b-containing neurons, a population that consists of central chemoreceptors (the ccRTN neurons) and blood pressure (BP)-regulating neurons (the C1 cells). The transfected neurons were activated with pulses of laser light. Respiratory effects were measured by plethysmography or diaphragmatic EMG recording and cardiovascular effects by monitoring BP, renal sympathetic nerve discharge, and the baroreflex.
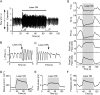
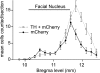
Measurements and main results: The RVLM contained 600 to 900 ChR2-transfected neurons (63% C1, 37% ccRTN). RVLM photostimulation significantly increased breathing rate (+42%), tidal volume (21%), minute volume (68%), and peak expiratory flow (48%). Photostimulation increased diaphragm EMG amplitude (19%) and frequency (21%). Photostimulation increased BP (4 mmHg) and renal sympathetic nerve discharge (43%) while decreasing heart rate (15 bpm).
Conclusions: Photostimulation of ChR2-transfected RVLM Phox2b neurons produces a vigorous stimulation of breathing accompanied by a small sympathetically mediated increase in BP. These results demonstrate that breathing can be relatively selectively activated in resting unanesthetized mammals via optogenetic manipulation of RVLM neurons presumed to be central chemoreceptors. This methodology could perhaps be used in the future to enhance respiration in humans.
Figures

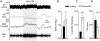

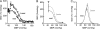

Similar articles
-
Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats.Am J Respir Crit Care Med. 2014 Dec 1;190(11):1301-10. doi: 10.1164/rccm.201407-1262OC. Am J Respir Crit Care Med. 2014. PMID: 25325789 Free PMC article.
-
Differential Contribution of the Retrotrapezoid Nucleus and C1 Neurons to Active Expiration and Arousal in Rats.J Neurosci. 2020 Nov 4;40(45):8683-8697. doi: 10.1523/JNEUROSCI.1006-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973046 Free PMC article.
-
Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats.J Physiol. 2009 Dec 1;587(Pt 23):5613-31. doi: 10.1113/jphysiol.2009.177535. Epub 2009 Oct 12. J Physiol. 2009. PMID: 19822543 Free PMC article.
-
The retrotrapezoid nucleus and breathing.Adv Exp Med Biol. 2012;758:115-22. doi: 10.1007/978-94-007-4584-1_16. Adv Exp Med Biol. 2012. PMID: 23080151 Free PMC article. Review.
-
Respiratory and autonomic dysfunction in congenital central hypoventilation syndrome.J Neurophysiol. 2016 Aug 1;116(2):742-52. doi: 10.1152/jn.00026.2016. Epub 2016 May 25. J Neurophysiol. 2016. PMID: 27226447 Free PMC article. Review.
Cited by
-
Optogenetic analysis of respiratory neuronal networks in the ventral medulla of neonatal rats producing channelrhodopsin in Phox2b-positive cells.Pflugers Arch. 2019 Dec;471(11-12):1419-1439. doi: 10.1007/s00424-019-02317-9. Epub 2019 Oct 20. Pflugers Arch. 2019. PMID: 31631251
-
Breathing responses produced by optogenetic stimulation of adrenergic C1 neurons are dependent on the connection with preBötzinger complex in rats.Pflugers Arch. 2018 Nov;470(11):1659-1672. doi: 10.1007/s00424-018-2186-0. Epub 2018 Jul 27. Pflugers Arch. 2018. PMID: 30054719
-
Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats.Respir Physiol Neurobiol. 2014 Jan 15;191:95-105. doi: 10.1016/j.resp.2013.11.005. Epub 2013 Nov 24. Respir Physiol Neurobiol. 2014. PMID: 24284037 Free PMC article.
-
TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons.J Neurosci. 2013 Oct 9;33(41):16033-44. doi: 10.1523/JNEUROSCI.2451-13.2013. J Neurosci. 2013. PMID: 24107938 Free PMC article.
-
Central chemoreceptors: locations and functions.Compr Physiol. 2012 Jan;2(1):221-54. doi: 10.1002/cphy.c100083. Compr Physiol. 2012. PMID: 23728974 Free PMC article. Review.
References
-
- Khong P, Lazzaro A, Mobbs R. Phrenic nerve stimulation: the Australian experience. J Clin Neurosci 2010;17:205–208. - PubMed
-
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol 2009;168:125–132. - PubMed
-
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 2010;181:626–644. - PubMed
-
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol 2001;129:247–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

