Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension
- PMID: 20622039
- PMCID: PMC3001258
- DOI: 10.1164/rccm.201001-0011OC
Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension
Abstract
Rationale: Prostacyclin analogs, used to treat idiopathic pulmonary arterial hypertension (IPAH), are assumed to work through prostacyclin (IP) receptors linked to cyclic AMP (cAMP) generation, although the potential to signal through peroxisome proliferator-activated receptor-γ (PPARγ) exists.
Objectives: IP receptor and PPARγ expression may be depressed in IPAH. We wished to determine if pathways remain functional and if analogs continue to inhibit smooth muscle proliferation.
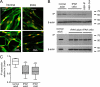
Methods: We used Western blotting to determine IP receptor expression in peripheral pulmonary arterial smooth muscle cells (PASMCs) from normal and IPAH lungs and immunohistochemistry to evaluate IP receptor and PPARγ expression in distal arteries.
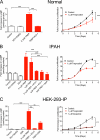
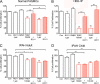
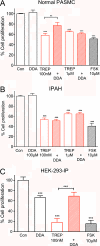
Measurements and main results: Cell proliferation and cAMP assays assessed analog responses in human and mouse PASMCs and HEK-293 cells. Proliferative rates of IPAH cells were greater than normal human PASMCs. IP receptor protein levels were lower in PASMCs from patients with IPAH, but treprostinil reduced replication and treprostinil-induced cAMP elevation appeared normal. Responses to prostacyclin analogs were largely dependent on the IP receptor and cAMP in normal PASMCs, although in IP(-/-) receptor cells analogs inhibited growth in a cAMP-independent, PPARγ-dependent manner. In IPAH cells, antiproliferative responses to analogs were insensitive to IP receptor or adenylyl cyclase antagonists but were potentiated by a PPARγ agonist and inhibited (∼ 60%) by the PPARγ antagonist GW9662. This coincided with increased PPARγ expression in the medial layer of acinar arteries.
Conclusions: The antiproliferative effects of prostacyclin analogs are preserved in IPAH despite IP receptor down-regulation and abnormal coupling. PPARγ may represent a previously unrecognized pathway by which these agents inhibit smooth muscle proliferation.
Figures



Similar articles
-
Pharmacology of the single isomer, esuberaprost (beraprost-314d) on pulmonary vascular tone, IP receptors and human smooth muscle proliferation in pulmonary hypertension.Biochem Pharmacol. 2019 Aug;166:242-252. doi: 10.1016/j.bcp.2019.05.026. Epub 2019 May 31. Biochem Pharmacol. 2019. PMID: 31158340
-
Prostanoid EP4 agonist L-902,688 activates PPARγ and attenuates pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2018 Mar 1;314(3):L349-L359. doi: 10.1152/ajplung.00245.2017. Epub 2017 Nov 16. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29146573
-
Role of the prostanoid EP4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension.Am J Respir Crit Care Med. 2008 Jul 15;178(2):188-96. doi: 10.1164/rccm.200710-1519OC. Epub 2008 May 8. Am J Respir Crit Care Med. 2008. PMID: 18467507
-
The prostacyclin pathway in pulmonary arterial hypertension: a clinical review.Expert Rev Respir Med. 2017 Jun;11(6):491-503. doi: 10.1080/17476348.2017.1317599. Epub 2017 Apr 24. Expert Rev Respir Med. 2017. PMID: 28399721 Review.
-
The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors.Prostaglandins Other Lipid Mediat. 2015 Jul;120:56-71. doi: 10.1016/j.prostaglandins.2015.04.007. Epub 2015 Apr 23. Prostaglandins Other Lipid Mediat. 2015. PMID: 25917921 Review.
Cited by
-
Prostanoid therapies in the management of pulmonary arterial hypertension.Ther Clin Risk Manag. 2015 Mar 31;11:535-47. doi: 10.2147/TCRM.S75122. eCollection 2015. Ther Clin Risk Manag. 2015. PMID: 25848300 Free PMC article. Review.
-
Prostanoid Signaling in Cancers: Expression and Regulation Patterns of Enzymes and Receptors.Biology (Basel). 2022 Apr 13;11(4):590. doi: 10.3390/biology11040590. Biology (Basel). 2022. PMID: 35453789 Free PMC article.
-
Novel Therapeutic Approaches of Pulmonary Arterial Hypertension.Int J Angiol. 2019 Jun;28(2):112-117. doi: 10.1055/s-0039-1692140. Epub 2019 Jul 12. Int J Angiol. 2019. PMID: 31384108 Free PMC article.
-
Mitochondrial dysfunction and pulmonary hypertension: cause, effect, or both.Am J Physiol Lung Cell Mol Physiol. 2018 May 1;314(5):L782-L796. doi: 10.1152/ajplung.00331.2017. Epub 2018 Jan 18. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29345195 Free PMC article. Review.
-
The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 1;308(3):L229-52. doi: 10.1152/ajplung.00238.2014. Epub 2014 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25416383 Free PMC article. Review.
References
-
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115:343–349. - PubMed
-
- Sandoval J, Bauerle O, Gomez A, Palomar A, Martinez Guerra ML, Furuya ME. Primary pulmonary hypertension in children: clinical characterization and survival. J Am Coll Cardiol 1995;25:466–474. - PubMed
-
- Rhodes CJ, Davidson A, Gibbs JS, Wharton J, Wilkins MR. Therapeutic targets in pulmonary arterial hypertension. Pharmacol Ther 2009;121:69–88. - PubMed
-
- Schermuly RT, Yilmaz H, Ghofrani HA, Woyda K, Pullamsetti S, Schulz A, Gessler T, Dumitrascu R, Weissmann N, Grimminger F, et al. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am J Respir Crit Care Med 2005;172:358–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

