Multicenter evaluation of infant lung function tests as cystic fibrosis clinical trial endpoints
- PMID: 20622043
- PMCID: PMC3159081
- DOI: 10.1164/rccm.200908-1236OC
Multicenter evaluation of infant lung function tests as cystic fibrosis clinical trial endpoints
Abstract
Rationale: The conducting of clinical trials in infants with cystic fibrosis (CF) has been hindered by lack of sensitive outcome measures.
Objectives: To evaluate safety, feasibility, and ability to detect abnormalities in lung function of serial pulmonary function tests (PFTs) in infants with CF.
Methods: Multicenter observational study using a commercial device, rigorous training, ongoing quality control, and over-reading of data by an independent panel. Raised volume rapid thoracoabdominal compression technique and plethysmography were performed at enrollment and at 6 and 12 months, with an additional 1-month reproducibility visit.
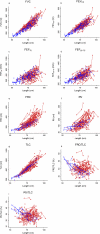
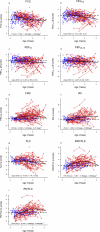
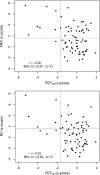
Measurements and main results: A total of 342 procedures were performed in 100 infants with CF at 10 centers. FRC measurements were acceptable at a higher proportion of study visits (89%) than raised volume (72%) or fractional lung volume (68%) measurements. Average Z scores for many parameters differed significantly from historical control values. Mean (95% confidence interval) Z scores were: -0.52 (-0.78 to -0.25) for forced expiratory flow at 75% (FEF₇₅) for FVC; 1.92 (1.39-2.45) for FRC; 1.22 (0.68-1.76) for residual volume; 0.87 (0.60-1.13) for FRC/total lung capacity; and 0.66 (0.27-1.06) for residual volume/total lung capacity. For future multicenter clinical trials using infant PFTs as primary endpoints, minimum detectable treatment effects are presented for several sample sizes.
Conclusions: In this 10-center study, key PFT measures were significantly different in infants with CF than in historical control subjects. However, infant PFTs do not yet appear ready as primary efficacy endpoints for multicenter clinical trials, particularly at inexperienced sites, based on acceptability rates, variability, and potentially large sample sizes required to detect reasonable treatment effects.
Figures
Comment in
-
Need for healthy control subjects when assessing lung function in infants with respiratory disease.Am J Respir Crit Care Med. 2010 Dec 1;182(11):1340-2. doi: 10.1164/rccm.201008-1338ED. Am J Respir Crit Care Med. 2010. PMID: 21123748 No abstract available.
References
-
- Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. for the, Group LCCF. Airway function in infants newly diagnosed with cystic fibrosis. Lancet 2001;358:1964–1965. - PubMed
-
- Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, Castle R, Dinwiddie R, Hoo AF, Lum S, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med 2004;169:928–933. - PubMed
-
- Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med 2008;178:1238–1244. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

