In vivo assessment of artery smooth muscle [Ca2+]i and MLCK activation in FRET-based biosensor mice
- PMID: 20622107
- PMCID: PMC2944472
- DOI: 10.1152/ajpheart.00359.2010
In vivo assessment of artery smooth muscle [Ca2+]i and MLCK activation in FRET-based biosensor mice
Abstract
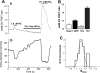
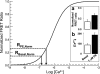
The cellular mechanisms that control arterial diameter in vivo, particularly in hypertension, are uncertain. Here, we report a method that permits arterial intracellular Ca(2+) concentration ([Ca(2+)](i)), myosin light-chain kinase (MLCK) activation, and artery external diameter to be recorded simultaneously with arterial blood pressure (BP) in living mice under 1.5% isofluorane anesthesia. The method also enables an assessment of local receptor activity on [Ca(2+)](i), MLCK activity, and diameter in arteries, uncomplicated by systemic effects. Transgenic mice that express, in smooth muscle, a Ca(2+)/calmodulin-activated, Förster resonance energy transfer (FRET)-based "ratiometric", exogenous MLCK biosensor were used. Vasoactive substances were administered either intravenously or locally to segments of exposed femoral or cremaster arteries. In the basal state, mean BP was approximately 90 mmHg, femoral arteries were constricted to 65% of their passive diameter, MLCK fractional activation was 0.14, and [Ca(2+)](i) was 131 nM. Phenylephrine (300 ng/g wt iv) elevated mean BP transiently to approximately 110 mmHg, decreased heart rate, increased femoral artery [Ca(2+)](i) to 244 nM and fractional MLCK activation to 0.24, and decreased artery diameter by 23%. In comparison, local application of 1.0 muM phenylephrine raised [Ca(2+)](i) to 279 nM and fractional MLCK activation to 0.26, and reduced diameter by 25%, but did not affect BP or heart rate. Intravital FRET imaging of exogenous MLCK biosensor mice permits quantification of changes in [Ca(2+)](i) and MLCK activation that accompany small changes in BP. Based on the observed variance of the FRET data, this method should enable the detection of a difference in basal [Ca(2+)](i) of 29 nM between two groups of 12 mice with a significance of P < 0.05.
Figures





References
-
- Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006 - PubMed
-
- Brekke JF, Jackson WF, Segal SS. Arteriolar smooth muscle Ca2+ dynamics during blood flow control in hamster cheek pouch. J Appl Physiol 101: 307–315, 2006 - PubMed
-
- Chen Y, Rivers RJ. Measurement of membrane potential and intracellular Ca(2+) of arteriolar endothelium and smooth muscle in vivo. Microvasc Res 62: 55–62, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

