Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats
- PMID: 20622169
- PMCID: PMC3279561
- DOI: 10.2337/db09-1564
Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats
Abstract
Objective: The efficacy of liraglutide, a human glucagon-like peptide-1 (GLP-1) analog, to prevent or delay diabetes in UCD-T2DM rats, a model of polygenic obese type 2 diabetes, was investigated.
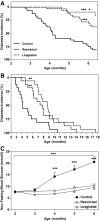
Research design and methods: At 2 months of age, male rats were divided into three groups: control, food-restricted, and liraglutide. Animals received liraglutide (0.2 mg/kg s.c.) or vehicle injections twice daily. Restricted rats were food restricted to equalize body weights to liraglutide-treated rats. Half of the animals were followed until diabetes onset, whereas the other half of the animals were killed at 6.5 months of age for tissue collection.
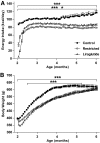
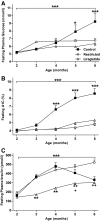
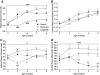
Results: Before diabetes onset energy intake, body weight, adiposity, and liver triglyceride content were higher in control animals compared with restricted and liraglutide-treated rats. Energy-restricted animals had lower food intake than liraglutide-treated animals to maintain the same body weights, suggesting that liraglutide increases energy expenditure. Liraglutide treatment delayed diabetes onset by 4.1 ± 0.8 months compared with control (P < 0.0001) and by 1.3 ± 0.8 months compared with restricted animals (P < 0.05). Up to 6 months of age, energy restriction and liraglutide treatment lowered fasting plasma glucose and A1C concentrations compared with control animals. In contrast, liraglutide-treated animals exhibited lower fasting plasma insulin, glucagon, and triglycerides compared with both control and restricted animals. Furthermore, energy-restricted and liraglutide-treated animals exhibited more normal islet morphology.
Conclusions: Liraglutide treatment delays the development of diabetes in UCD-T2DM rats by reducing energy intake and body weight, and by improving insulin sensitivity, improving lipid profiles, and maintaining islet morphology.
Figures

References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 - PubMed
-
- Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 2007;81:761–767 - PubMed
-
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 1987;2:1300–1304 - PubMed
-
- Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun 2009;390:613–618 - PubMed

