Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates
- PMID: 20622174
- PMCID: PMC3279532
- DOI: 10.2337/db10-0136
Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates
Abstract
Objective: To test the graft-promoting effects of mesenchymal stem cells (MSCs) in a cynomolgus monkey model of islet/bone marrow transplantation.
Research design and methods: Cynomolgus MSCs were obtained from iliac crest aspirate and characterized through passage 11 for phenotype, gene expression, differentiation potential, and karyotype. Allogeneic donor MSCs were cotransplanted intraportally with islets on postoperative day (POD) 0 and intravenously with donor marrow on PODs 5 and 11. Recipients were followed for stabilization of blood glucose levels, reduction of exogenous insulin requirement (EIR), C-peptide levels, changes in peripheral blood T regulatory cells, and chimerism. Destabilization of glycemia and increases in EIR were used as signs of rejection; additional intravenous MSCs were administered to test the effect on reversal of rejection.
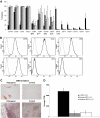
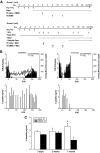
Results: MSC phenotype and a normal karyotype were observed through passage 11. IL-6, IL-10, vascular endothelial growth factor, TGF-β, hepatocyte growth factor, and galectin-1 gene expression levels varied among donors. MSC treatment significantly enhanced islet engraftment and function at 1 month posttransplant (n = 8), as compared with animals that received islets without MSCs (n = 3). Additional infusions of donor or third-party MSCs resulted in reversal of rejection episodes and prolongation of islet function in two animals. Stable islet allograft function was associated with increased numbers of regulatory T-cells in peripheral blood.
Conclusions: MSCs may provide an important approach for enhancement of islet engraftment, thereby decreasing the numbers of islets needed to achieve insulin independence. Furthermore, MSCs may serve as a new, safe, and effective antirejection therapy.
Figures




References
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736 - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 - PubMed
-
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002;30:42–48 - PubMed
-
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–3843 - PubMed
-
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722–3729 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

