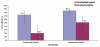Performance of rotavirus vaccines in developed and developing countries
- PMID: 20622508
- PMCID: PMC3322519
- DOI: 10.4161/hv.6.7.11278
Performance of rotavirus vaccines in developed and developing countries
Abstract
The World Health Organization estimates that rotavirus diarrhea results in approximately half a million deaths and approximately 2.4 million hospitalizations in developing countries each year. Two live oral rotavirus vaccines, RotaTeq® (RV 5; Merck) and Rotarix® (RV 1; GlaxoSmithKline) with good efficacy against severe rotavirus disease and a reassuring safety profile could substantially impact the burden of rotavirus disease. In April 2009, WHO provided a recommendation for global introduction of these vaccines in national immunization programs of developing countries worldwide. In this article, we review published data on previous candidate rotavirus vaccines and vaccines in current use, with emphasis on their performance in developed versus developing countries. In developed countries, both first and second generation rotavirus vaccines have demonstrated high efficacy against severe rotavirus disease (pooled efficacy = 73% and 85%, respectively). In developing countries, small early trials for the first generation vaccines failed to provide protection against rotavirus disease (pooled efficacy = 20%), however, trials of the second generation vaccines yielded substantial improvements in efficacy in developing countries (pooled efficacy of 51%), leading to a global recommendation for rotavirus vaccine introduction by WHO. Future efforts for these vaccines should focus on optimizing the efficacy and delivery of these vaccines in challenging target populations of Asia and Africa with the greatest burden of severe rotavirus disease.
Figures
References
-
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:9–15. - PubMed
-
- Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. - PubMed
-
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous