Time-resolved FRET between GPCR ligands reveals oligomers in native tissues
- PMID: 20622858
- PMCID: PMC3506176
- DOI: 10.1038/nchembio.396
Time-resolved FRET between GPCR ligands reveals oligomers in native tissues
Abstract
G protein-coupled receptor (GPCR) oligomers have been proposed to play critical roles in cell signaling, but confirmation of their existence in a native context remains elusive, as no direct interactions between receptors have been reported. To demonstrate their presence in native tissues, we developed a time-resolved FRET strategy that is based on receptor labeling with selective fluorescent ligands. Specific FRET signals were observed with four different receptors expressed in cell lines, consistent with their dimeric or oligomeric nature in these transfected cells. More notably, the comparison between FRET signals measured with sets of fluorescent agonists and antagonists was consistent with an asymmetric relationship of the two protomers in an activated GPCR dimer. Finally, we applied the strategy to native tissues and succeeded in demonstrating the presence of oxytocin receptor dimers and/or oligomers in mammary gland.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures


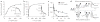
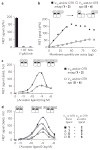
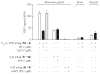
Comment in
-
Microscopy: GPCR dimers moving closer.Nat Chem Biol. 2010 Aug;6(8):570-1. doi: 10.1038/nchembio.409. Nat Chem Biol. 2010. PMID: 20644543 No abstract available.
References
-
- Fotiadis D, et al. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–259. - PubMed
-
- Wreggett KA, Wells JW. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem. 1995;270:22488–22499. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

