Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne
- PMID: 20622874
- PMCID: PMC2917782
- DOI: 10.1038/nsmb.1873
Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne
Abstract
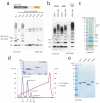
Ubiquitin is a versatile cellular signaling molecule that can form polymers of eight different linkages, and individual linkage types have been associated with distinct cellular functions. Though little is currently known about Lys11-linked ubiquitin chains, recent data indicate that they may be as abundant as Lys48 linkages and may be involved in vital cellular processes. Here we report the generation of Lys11-linked polyubiquitin in vitro, for which the Lys11-specific E2 enzyme UBE2S was fused to a ubiquitin binding domain. Crystallographic and NMR analyses of Lys11-linked diubiquitin reveal that Lys11-linked chains adopt compact conformations in which Ile44 is solvent exposed. Furthermore, we identify the OTU family deubiquitinase Cezanne as the first deubiquitinase with Lys11-linkage preference. Our data highlight the intrinsic specificity of the ubiquitin system that extends to Lys11-linked chains and emphasize that differentially linked polyubiquitin chains must be regarded as independent post-translational modifications.
Figures
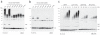





References
Experimental Procedures References
-
- Pickart CM, Raasi S. Controlled synthesis of polyubiquitin chains. Methods Enzymol. 2005;399:21–36. - PubMed
-
- Mori S, Abeygunawardana C, Johnson MO, van Zijl PC. Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J Magn Reson B. 1995;108:94–8. - PubMed
-
- Hajduk PJ, et al. NMR-based discovery of lead inhibitors that block DNA binding of the human papillomavirus E2 protein. J Med Chem. 1997;40:3144–50. - PubMed
-
- Evans P. Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr. 2006;62:72–82. - PubMed
References
-
- Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37:937–53. - PubMed
-
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–86. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. - PubMed
-
- Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–6. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

