Perturbation of the developmental potential of preimplantation mouse embryos by hydroxyurea
- PMID: 20623009
- PMCID: PMC2898034
- DOI: 10.3390/ijerph7052033
Perturbation of the developmental potential of preimplantation mouse embryos by hydroxyurea
Abstract
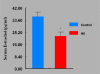
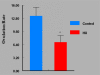
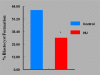
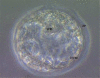
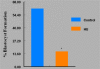
Women are advised not to attempt pregnancy while on hydroxyurea (HU) due to the teratogenic effects of this agent, based on results obtained from animal studies. Several case reports suggest that HU may have minimal or no teratogenic effects on the developing human fetus. Fourteen cases of HU therapy in pregnant patients diagnosed with acute or chronic myelogenous leukemia, primary thrombocythemia, or sickle cell disease (SCD) have been reported. Three pregnancies were terminated by elective abortion; 1 woman developed eclampsia and delivered a phenotypically normal stillborn infant. All other patients delivered live, healthy infants without congenital anomalies. We contend that case studies such as these have too few patients and cannot effectively address the adverse effect of HU on preimplantation embryo or fetuses. The objective of this study was to assess the risks associated with a clinically relevant dose of HU used for the treatment of SCD, on ovulation rate and embryo development, using adult C57BL/6J female mice as a model. In Experiment 1, adult female mice were randomly assigned to a treatment or a control group (N = 20/group). Treatment consisted of oral HU (30 mg/kg) for 28 days; while control mice received saline (HU vehicle). Five days to the cessation of HU dosing, all mice were subjected to folliculogenesis induction with pregnant mare serum gonadotropin (PMSG). Five mice/group were anesthetized at 48 hours post PMSG to facilitate blood collection via cardiac puncture for estradiol-17beta (E(2)) measurement by RIA. Ovulation was induced in the remaining mice at 48 hours post PMSG with human chorionic gonadotropin (hCG) and immediately caged with adult males for mating. Five plugged female mice/group were sacrificed for the determination of ovulation rate. The remaining mated mice were sacrificed about 26 hours post hCG, ovaries excised and weighed and embryos harvested and cultured in Whitten's medium (WM) supplemented with CZBt. In Experiments 2 and 3, (N = 10/Experiment) folliculogenesis and ovulation were induced in untreated mice followed by mating. Recovered embryos were either exposed continuously (Experiment 2) or intermittently (Experiment 3) to bioavailable HU (18 microg HU/mL of WM + CZBt) or WM + CZBt only (control). Treated mice sustained decreased ovarian wt, ovulation rate and circulating E(2) compared with controls (P < 0.05). Fewer embryos retrieved from HU-treated mice developed to blastocyst stage (32%) compared with those from controls (60%; P < 0.05). Furthermore, continuous or intermittent in vitro exposures of embryos to HU also resulted in reduced development to blastocyst stage (continuous HU, 9 vs. control, 63%; P < 0.05; intermittent HU, 20 vs. control, 62%; P < 0.05) with embryos exposed continuously to HU in vitro fairing worse. Even though HU is well tolerated, our data suggest that it compromises folliculogenesis and the ability of generated embryos to develop. Therefore, designed studies with larger numbers of patients receiving HU during pregnancy, with longer follow-up of exposed children and more careful assessment of embryo/fetotoxic effects, are required before this agent can be promoted as safe in pregnancy.
Keywords: 2-cell embryo; blastocyst; estradiol-17β ovulation rate; hydroxyurea; ovarian weight.
Figures
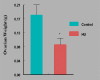

References
-
- Yarbro JW. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19:1–10. - PubMed
-
- Caramazza D, Caracciolo C, Barone R, Malato A, Saccullo G, Cigna V, Berretta S, Schinocca L, Quintini G, Abbadessa V, Di Raimondo F, Siragusa S. Correlation between leukocytosis and thrombosis in Philadelphia-negative chronic myeloprolipherative neoplasm. Ann. Hematol. 2009;88:967–971. - PubMed
-
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, Barbui T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N. Engl. J. Med. 1995;332:1132–1136. - PubMed
-
- Silver RT, Woolf SH, Hehlmann R, Appelbaum FR, Anderson J, Bennett C, Goldman JM, Guilhot F, Kantarjian HM, Lichtin AE, Talpaz M, Tura S. An evidence-based analysis of the effect of busulfan,hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood. 1999;94:1517–1536. - PubMed
-
- Steinberg MH. Management of sickle cell disease. N. Engl. J. Med. 1999;340:1021–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

