Adverse drug events in the outpatient setting: an 11-year national analysis
- PMID: 20623513
- PMCID: PMC2932855
- DOI: 10.1002/pds.1984
Adverse drug events in the outpatient setting: an 11-year national analysis
Abstract
Purpose: Adverse drug events (ADEs) are a common complication of medical care resulting in high morbidity and medical expenditure. Population level estimates of outpatient ADEs are limited. Our objective was to provide national estimates and characterizations of outpatient ADEs and determine risk factors associated with these events.
Methods: Data are from the National Center for Health Statistics which collects information on patient visits to outpatient clinics and emergency departments throughout the United States. We examined visits between 1995 and 2005 and measured the national annual estimates of and risk factors for outpatient ADEs requiring medical treatment.
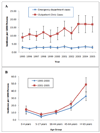
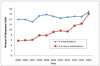
Results: The national annual number of ADE-related visits was 4 335,990 (95%CI: 4 326 872-4 345 108). Visits for ADEs to outpatient clinics increased over the study period from 9.0 to 17.0 per 1000 persons (p-value for trend < 0.001). In multivariate analyses, factors associated with ADE visits included patient age (OR: 2.13; 95%CI: 1.63-2.79 for 65 years and older), number of medications taken by patient (OR: 1.88; 95%CI: 1.58-2.25 for five medications or more), and female gender (OR: 1.51; 95%CI: 1.34-1.71). Overall, outpatient ADEs resulted in 107,468 (95%CI: 89 011-125 925) hospital admissions annually, with older patients at highest risk for hospitalization (p-value for trend < 0.001).
Conclusions: Both patient age and polypharmacy use are risk factors for ADE-related healthcare visits, which have substantially increased in outpatient clinics between 1995 and 2005. The incidence of ADEs has particularly increased among patients 65 years and older with as many as 1 in 20 persons seeking medical care for an ADE.
(c) 2010 John Wiley & Sons, Ltd.
Figures
References
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Jama. 1998;279(15):1200–1205. - PubMed
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama. 1995;274(1):29–34. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. Jama. 1997;277(4):301–306. - PubMed
-
- Mjorndal T, Boman MD, Hagg S, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11(1):65–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

