Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype
- PMID: 20624947
- PMCID: PMC2916954
- DOI: 10.4049/jimmunol.1000818
Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype
Abstract
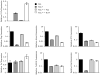
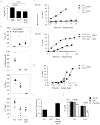
CD8 T cells can acquire cytokine-secreting phenotypes paralleling cytokine production from Th cells. IL-17-secreting CD8 T cells, termed Tc17 cells, were shown to promote inflammation and mediate immunity to influenza. However, most reports observed a lack of cytotoxic activity by Tc17 cells. In this study, we explored the anti-viral activity of Tc17 cells using a vaccinia virus (VV) infection model. Tc17 cells expanded during VV infection, and TCR transgenic Tc17 cells were capable of clearing recombinant VV infection. In vivo, adoptively transferred Tc17 cells lost the IL-17-secreting phenotype, even in the absence of stimulation, but they did not acquire IFN-gamma-secreting potential unless stimulated with a virus-encoded Ag. However, examination of cells following infection demonstrated that these cells acquired cytotoxic potential in vivo, even in the absence of IFN-gamma. Cytotoxic potential correlated with Fasl expression, and the cytotoxic activity of postinfection Tc17 cells was partially blocked by the addition of anti-FasL. Thus, Tc17 cells mediate VV clearance through expression of specific molecules associated with cytotoxicity but independent of an acquired Tc1 phenotype.
Figures





References
-
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. - PubMed
-
- Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. - PubMed
-
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. - PubMed
-
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. - PubMed
-
- Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

