Tissue kallikrein permits early renal adaptation to potassium load
- PMID: 20624970
- PMCID: PMC2922146
- DOI: 10.1073/pnas.0913070107
Tissue kallikrein permits early renal adaptation to potassium load
Abstract
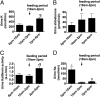
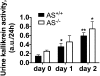
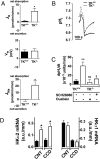
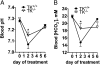
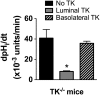
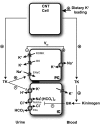
Tissue kallikrein (TK) is a serine protease synthetized in renal tubular cells located upstream from the collecting duct where renal potassium balance is regulated. Because secretion of TK is promoted by K+ intake, we hypothesized that this enzyme might regulate plasma K+ concentration ([K+]). We showed in wild-type mice that renal K+ and TK excretion increase in parallel after a single meal, representing an acute K+ load, whereas aldosterone secretion is not modified. Using aldosterone synthase-deficient mice, we confirmed that the control of TK secretion is aldosterone-independent. Mice with TK gene disruption (TK-/-) were used to assess the impact of the enzyme on plasma [K+]. A single large feeding did not lead to any significant change in plasma [K+] in TK+/+, whereas TK-/- mice became hyperkalemic. We next examined the impact of TK disruption on K+ transport in isolated cortical collecting ducts (CCDs) microperfused in vitro. We found that CCDs isolated from TK-/- mice exhibit net transepithelial K+ absorption because of abnormal activation of the colonic H+,K+-ATPase in the intercalated cells. Finally, in CCDs isolated from TK-/- mice and microperfused in vitro, the addition of TK to the perfusate but not to the peritubular bath caused a 70% inhibition of H+,K+-ATPase activity. In conclusion, we have identified the serine protease TK as a unique kalliuretic factor that protects against hyperkalemia after a dietary K+ load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
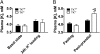
References
-
- Giebisch G. Renal potassium transport: Mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. - PubMed
-
- Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. - PubMed
-
- Young DB, Paulsen AW. Interrelated effects of aldosterone and plasma potassium on potassium excretion. Am J Physiol. 1983;244:F28–F34. - PubMed
-
- Young DB. Quantitative analysis of aldosterone's role in potassium regulation. Am J Physiol. 1988;255:F811–F822. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

