Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury
- PMID: 20624979
- PMCID: PMC2922128
- DOI: 10.1073/pnas.1001815107
Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury
Abstract
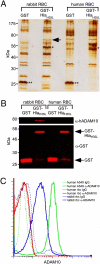
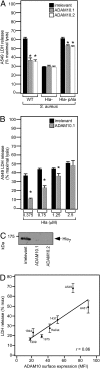
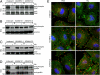
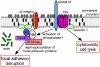
Staphylococcus aureus alpha-hemolysin (Hla), a potent cytotoxin, plays an important role in the pathogenesis of staphylococcal diseases, including those caused by methicillin-resistant epidemic strains. Hla is secreted as a water-soluble monomer that undergoes a series of conformational changes to generate a heptameric, beta-barrel structure in host membranes. Structural maturation of Hla depends on its interaction with a previously unknown proteinaceous receptor in the context of the cell membrane. It is reported here that a disintegrin and metalloprotease 10 (ADAM10) interacts with Hla and is required to initiate the sequence of events whereby the toxin is transformed into a cytolytic pore. Hla binding to the eukaryotic cell requires ADAM10 expression. Further, ADAM10 is required for Hla-mediated cytotoxicity, most notably when the toxin is present at low concentrations. These data thus implicate ADAM10 as the probable high-affinity toxin receptor. Upon Hla binding, ADAM10 relocalizes to caveolin 1-enriched lipid rafts that serve as a platform for the clustering of signaling molecules. It is demonstrated that the Hla-ADAM10 complex initiates intracellular signaling events that culminate in the disruption of focal adhesions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

