Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis
- PMID: 20625009
- PMCID: PMC2996109
- DOI: 10.1182/blood-2010-01-261503
Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis
Abstract
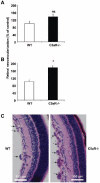
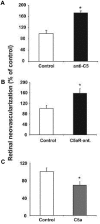
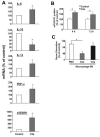
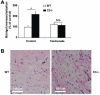
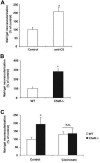
Beyond its role in immunity, complement mediates a wide range of functions in the context of morphogenetic or tissue remodeling processes. Angiogenesis is crucial during tissue remodeling in multiple pathologies; however, the knowledge about the regulation of neovascularization by the complement components is scarce. Here we studied the involvement of complement in pathological angiogenesis. Strikingly, we found that mice deficient in the central complement component C3 displayed increased neovascularization in the model of retinopathy of prematurity (ROP) and in the in vivo Matrigel plug assay. In addition, antibody-mediated blockade of C5, treatment with C5aR antagonist, or C5aR deficiency in mice resulted in enhanced pathological retina angiogenesis. While complement did not directly affect angiogenesis-related endothelial cell functions, we found that macrophages mediated the antiangiogenic activity of complement. In particular, C5a-stimulated macrophages were polarized toward an angiogenesis-inhibitory phenotype, including the up-regulated secretion of the antiangiogenic soluble vascular endothelial growth factor receptor-1. Consistently, macrophage depletion in vivo reversed the increased neovascularization associated with C3- or C5aR deficiency. Taken together, complement and in particular the C5a-C5aR axes are potent inhibitors of angiogenesis.
Figures

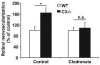
Comment in
-
Complement halts angiogenesis gone wild.Blood. 2010 Nov 25;116(22):4393-4. doi: 10.1182/blood-2010-08-297648. Blood. 2010. PMID: 21109626 No abstract available.
References
-
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. - PubMed
-
- Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. - PubMed
-
- Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30(2):83–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

