Cholesteryl ester hydrolase activity is abolished in HSL-/- macrophages but unchanged in macrophages lacking KIAA1363
- PMID: 20625037
- PMCID: PMC2936755
- DOI: 10.1194/jlr.M004259
Cholesteryl ester hydrolase activity is abolished in HSL-/- macrophages but unchanged in macrophages lacking KIAA1363
Abstract
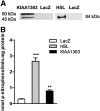
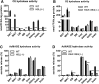
Cholesteryl ester (CE) accumulation in macrophages represents a crucial event during foam cell formation, a hallmark of atherogenesis. Here we investigated the role of two previously described CE hydrolases, hormone-sensitive lipase (HSL) and KIAA1363, in macrophage CE hydrolysis. HSL and KIAA1363 exhibited marked differences in their abilities to hydrolyze CE, triacylglycerol (TG), diacylglycerol (DG), and 2-acetyl monoalkylglycerol ether (AcMAGE), a precursor for biosynthesis of platelet-activating factor (PAF). HSL efficiently cleaved all four substrates, whereas KIAA1363 hydrolyzed only AcMAGE. This contradicts previous studies suggesting that KIAA1363 is a neutral CE hydrolase. Macrophages of KIAA1363(-/-) and wild-type mice exhibited identical neutral CE hydrolase activity, which was almost abolished in tissues and macrophages of HSL(-/-) mice. Conversely, AcMAGE hydrolase activity was diminished in macrophages and some tissues of KIAA1363(-/-) but unchanged in HSL(-/-) mice. CE turnover was unaffected in macrophages lacking KIAA1363 and HSL, whereas cAMP-dependent cholesterol efflux was influenced by HSL but not by KIAA1363. Despite decreased CE hydrolase activities, HSL(-/-) macrophages exhibited CE accumulation similar to wild-type (WT) macrophages. We conclude that additional enzymes must exist that cooperate with HSL to regulate CE levels in macrophages. KIAA1363 affects AcMAGE hydrolase activity but is of minor importance as a direct CE hydrolase in macrophages.
Figures






References
-
- Brown M. S., Ho Y. K., Goldstein J. L. 1980. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 255: 9344–9352. - PubMed
-
- Ho Y. K., Brown M. S., Goldstein J. L. 1980. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J. Lipid Res. 21: 391–398. - PubMed
-
- Goodman D. S. 1965. Cholesterol ester metabolism. Physiol. Rev. 45: 747–839. - PubMed
-
- Small C. A., Goodacre J. A., Yeaman S. J. 1989. Hormone-sensitive lipase is responsible for the neutral cholesterol ester hydrolase activity in macrophages. FEBS Lett. 247: 205–208. - PubMed
-
- Escary J. L., Choy H. A., Reue K., Schotz M. C. 1998. Hormone-sensitive lipase overexpression increases cholesteryl ester hydrolysis in macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 18: 991–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

