The role of nucleosome positioning in the evolution of gene regulation
- PMID: 20625544
- PMCID: PMC2897762
- DOI: 10.1371/journal.pbio.1000414
The role of nucleosome positioning in the evolution of gene regulation
Abstract
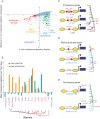
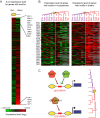
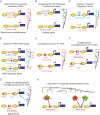
Chromatin organization plays a major role in gene regulation and can affect the function and evolution of new transcriptional programs. However, it can be difficult to decipher the basis of changes in chromatin organization and their functional effect on gene expression. Here, we present a large-scale comparative genomic analysis of the relationship between chromatin organization and gene expression, by measuring mRNA abundance and nucleosome positions genome-wide in 12 Hemiascomycota yeast species. We found substantial conservation of global and functional chromatin organization in all species, including prominent nucleosome-free regions (NFRs) at gene promoters, and distinct chromatin architecture in growth and stress genes. Chromatin organization has also substantially diverged in both global quantitative features, such as spacing between adjacent nucleosomes, and in functional groups of genes. Expression levels, intrinsic anti-nucleosomal sequences, and trans-acting chromatin modifiers all play important, complementary, and evolvable roles in determining NFRs. We identify five mechanisms that couple chromatin organization to evolution of gene regulation and have contributed to the evolution of respiro-fermentation and other key systems, including (1) compensatory evolution of alternative modifiers associated with conserved chromatin organization, (2) a gradual transition from constitutive to trans-regulated NFRs, (3) a loss of intrinsic anti-nucleosomal sequences accompanying changes in chromatin organization and gene expression, (4) re-positioning of motifs from NFRs to nucleosome-occluded regions, and (5) the expanded use of NFRs by paralogous activator-repressor pairs. Our study sheds light on the molecular basis of chromatin organization, and on the role of chromatin organization in the evolution of gene regulation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- King M. C, Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–116. - PubMed
-
- McAdams H. H, Srinivasan B, Arkin A. P. The evolution of genetic regulatory systems in bacteria. Nat Rev Genet. 2004;5:169–178. - PubMed
-
- Gasch A. P, Moses A. M, Chiang D. Y, Fraser H. B, Berardini M, et al. Conservation and evolution of cis-regulatory systems in ascomycete fungi. PLoS Biol. 2004;2:e398. doi: 10.1371/journal.pbio.0020398. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

