Thiol-based redox switches and gene regulation
- PMID: 20626317
- PMCID: PMC3113447
- DOI: 10.1089/ars.2010.3400
Thiol-based redox switches and gene regulation
Abstract
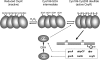
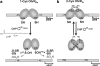
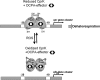
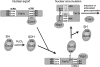
Cysteine is notable among the universal, proteinogenic amino acids for its facile redox chemistry. Cysteine thiolates are readily modified by reactive oxygen species (ROS), reactive electrophilic species (RES), and reactive nitrogen species (RNS). Although thiol switches are commonly triggered by disulfide bond formation, they can also be controlled by S-thiolation, S-alkylation, or modification by RNS. Thiol-based switches are common in both prokaryotic and eukaryotic organisms and activate functions that detoxify reactive species and restore thiol homeostasis while repressing functions that would be deleterious if expressed under oxidizing conditions. Here, we provide an overview of the best-understood examples of thiol-based redox switches that affect gene expression. Intra- or intermolecular disulfide bond formation serves as a direct regulatory switch for several bacterial transcription factors (OxyR, OhrR/2-Cys, Spx, YodB, CrtJ, and CprK) and indirectly regulates others (the RsrA anti-σ factor and RegB sensory histidine kinase). In eukaryotes, thiol-based switches control the yeast Yap1p transcription factor, the Nrf2/Keap1 electrophile and oxidative stress response, and the Chlamydomonas NAB1 translational repressor. Collectively, these regulators reveal a remarkable range of chemical modifications exploited by Cys residues to effect changes in gene expression.
Figures
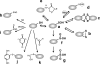




References
-
- Antelmann H. Hecker M. Zuber P. Proteomic signatures uncover thiol-specific electrophile resistance mechanisms in Bacillus subtilis. Expert Rev Proteom. 2008;5:77–90. - PubMed
-
- Bae JB. Park JH. Hahn MY. Kim MS. Roe JH. Redox-dependent changes in RsrA, an anti-sigma factor in Streptomyces coelicolor: zinc release and disulfide bond formation. J Mol Biol. 2004;335:425–435. - PubMed
-
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol. 2004;14:679–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

