Dysregulation of striatal dopamine release in a mouse model of dystonia
- PMID: 20626557
- PMCID: PMC2951331
- DOI: 10.1111/j.1471-4159.2010.06890.x
Dysregulation of striatal dopamine release in a mouse model of dystonia
Abstract
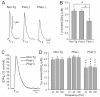
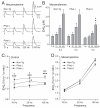
Dystonia is a neurological disorder characterized by involuntary movements. We examined striatal dopamine (DA) function in hyperactive transgenic (Tg) mice generated as a model of dystonia. Evoked extracellular DA concentration was monitored with carbon-fiber microelectrodes and fast-scan cyclic voltammetry in striatal slices from non-Tg mice, Tg mice with a positive motor phenotype, and phenotype-negative Tg littermates. Peak single-pulse evoked extracellular DA concentration was significantly lower in phenotype-positive mice than in non-Tg or phenotype-negative mice, but indistinguishable between non-Tg and phenotype-negative mice. Phenotype-positive mice also had higher functional D2 DA autoreceptor sensitivity than non-Tg mice, which would be consistent with lower extracellular DA concentration in vivo. Multiple-pulse (phasic) stimulation (five pulses, 10-100 Hz) revealed an enhanced frequency dependence of evoked DA release in phenotype-positive versus non-Tg or phenotype-negative mice, which was exacerbated when extracellular Ca(2+) concentration was lowered. Enhanced sensitivity to phasic stimulation in phenotype-positive mice was reminiscent of the pattern seen with antagonism of nicotinic acetylcholine receptors. Consistent with a role for altered cholinergic regulation, the difference in phasic responsiveness among groups was lost when nicotinic receptors were blocked by mecamylamine. Together, these data implicate compromised DA release regulation, possibly from cholinergic dysfunction, in the motor symptoms of this dystonia model.
© 2010 The Authors. Journal Compilation © 2010 International Society for Neurochemistry.
Figures


Similar articles
-
Characterization of Optically and Electrically Evoked Dopamine Release in Striatal Slices from Digenic Knock-in Mice with DAT-Driven Expression of Channelrhodopsin.ACS Chem Neurosci. 2017 Feb 15;8(2):310-319. doi: 10.1021/acschemneuro.6b00300. Epub 2017 Feb 8. ACS Chem Neurosci. 2017. PMID: 28177213 Free PMC article.
-
Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum.J Neurosci. 2001 Oct 1;21(19):7841-7. doi: 10.1523/JNEUROSCI.21-19-07841.2001. J Neurosci. 2001. PMID: 11567075 Free PMC article.
-
Function of dopamine transporter is compromised in DYT1 transgenic animal model in vivo.J Neurochem. 2010 Apr;113(1):228-35. doi: 10.1111/j.1471-4159.2010.06590.x. Epub 2010 Feb 2. J Neurochem. 2010. PMID: 20132487 Free PMC article.
-
Mice transgenic for exon 1 of Huntington's disease: properties of cholinergic and dopaminergic pre-synaptic function in the striatum.J Neurochem. 2003 May;85(4):1054-63. doi: 10.1046/j.1471-4159.2003.01704.x. J Neurochem. 2003. PMID: 12716437
-
Functional pathology of neuroleptic-induced dystonia based on the striatal striosome-matrix dopamine system in humans.J Neurol Neurosurg Psychiatry. 2025 Jan 16;96(2):177-183. doi: 10.1136/jnnp-2024-334545. J Neurol Neurosurg Psychiatry. 2025. PMID: 39631787 Review.
Cited by
-
Emerging common molecular pathways for primary dystonia.Mov Disord. 2013 Jun 15;28(7):968-81. doi: 10.1002/mds.25547. Mov Disord. 2013. PMID: 23893453 Free PMC article. Review.
-
Molecular pathways in dystonia.Neurobiol Dis. 2011 May;42(2):136-47. doi: 10.1016/j.nbd.2010.11.015. Epub 2010 Dec 4. Neurobiol Dis. 2011. PMID: 21134457 Free PMC article. Review.
-
Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures.J Neurosci Methods. 2011 Nov 15;202(2):158-64. doi: 10.1016/j.jneumeth.2011.03.001. Epub 2011 Mar 8. J Neurosci Methods. 2011. PMID: 21392532 Free PMC article.
-
The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain.Front Aging Neurosci. 2021 Jul 13;13:654931. doi: 10.3389/fnagi.2021.654931. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34326765 Free PMC article. Review.
-
Subsecond regulation of striatal dopamine release by pre-synaptic KATP channels.J Neurochem. 2011 Sep;118(5):721-36. doi: 10.1111/j.1471-4159.2011.07358.x. Epub 2011 Aug 4. J Neurochem. 2011. PMID: 21689107 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Augood SJ, Keller-McGandy CE, Siriani A, Hewett J, Ramesh V, Sapp E, DiFiglia M, Breakefield XO, Standaert DG. Distribution and ultrastructural localization of torsinA immunoreactivity in the human brain. Brain Res. 2003;986:12–21. - PubMed
-
- Augood SJ, Martin DM, Ozelius LJ, Breakefield XO, Penney JB, Jr., Standaert DG. Distribution of the mRNAs encoding torsinA and torsinB in the normal adult human brain. Ann Neurol. 1999;46:761–769. - PubMed
-
- Augood SJ, Penney JB, Jr, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, Standaert DG. Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann. Neurol. 1998;43:669–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

