Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis
- PMID: 20626559
- PMCID: PMC2939970
- DOI: 10.1111/j.1471-4159.2010.06898.x
Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis
Abstract
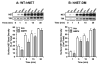
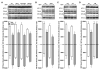
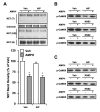
D-amphetamine (AMPH) down-regulates the norepinephrine transporter (NET), although the exact trafficking pathways altered and motifs involved are not known. Therefore, we examined the cellular and molecular mechanisms involved in AMPH-induced NET regulation in human placental trophoblast cells expressing the wild-type (WT)-hNET and the hNET double mutant (DM)-bearing protein kinase C (PKC)-resistant T258A + S259A motif. NET function and surface expression were significantly reduced in cells expressing WT-hNET but not in cells expressing hNET-DM following AMPH treatment. AMPH inhibited plasma membrane recycling of both WT-hNET and hNET-DM. In contrast, AMPH stimulated endocytosis of WT-hNET, and did not affect hNET-DM endocytosis. Although PKC or calcium/calmodulin- dependent kinase-II (CaMKII) inhibition or depletion of calcium failed to block AMPH-mediated down-regulation of WT-hNET, NET-specific blocker desipramine completely prevented AMPH-induced down-regulation. Furthermore, AMPH treatment had no effect on phospho-CaMKII immunoreactivity. The inhibitory potency of AMPH was highest on hNET-DM, intermediary on T258A and S259A single mutants and lowest on WT-hNET. Single mutants exhibited partial resistance to AMPH-mediated down-regulation. AMPH accumulation was similar in cells expressing WT-hNET or hNET-DM. The results demonstrate that reduced plasma membrane insertion and enhanced endocytosis account for AMPH-mediated NET down-regulation, and provide the first evidence that T258/S259 motif is involved only in AMPH-induced NET endocytosis that is desipramine-sensitive, but PKC and CaMKII independent.
© 2010 The Authors. Journal Compilation © 2010 International Society for Neurochemistry.
Figures





References
-
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998;287:733–743. - PubMed
-
- Aromolaran AA, Blatter LA. Modulation of intracellular Ca2+ release and capacitative Ca2+ entry by CaMKII inhibitors in bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2005;289:C1426–1436. - PubMed
-
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

