General aspects and neuropathology of X-linked adrenoleukodystrophy
- PMID: 20626743
- PMCID: PMC8094714
- DOI: 10.1111/j.1750-3639.2010.00390.x
General aspects and neuropathology of X-linked adrenoleukodystrophy
Abstract
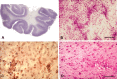
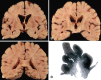
X-adrenoleukodystrophy (X-ALD) is a metabolic, peroxisomal disease affecting the nervous system, adrenal cortex and testis resulting from inactivating mutations in ABCD1 gene which encodes a peroxisomal membrane half-adenosine triphosphate (ATP)-binding cassette transporter, ABCD1 (or ALDP), whose defect is associated with impaired peroxisomal beta-oxidation and accumulation of saturated very long-chain fatty acids (VLCFA) in tissues and body fluids. Several phenotypes are recognized in male patients including cerebral ALD in childhood, adolescence or adulthood, adrenomyeloneuropathy (AMN), Addison's disease and, eventually, gonadal insufficiency. Female carriers might present with mild to severe myeloneuropathy that resembles AMN. There is a lack of phenotype-genotype correlations, as the same ABCD1 gene mutation may be associated with different phenotypes in the same family, suggesting that genetic, epigenetic, environmental and stochastic factors are probably contributory to the development and course of the disease. Degenerative changes, like those seen in pure AMN without cerebral demyelination, are characterized by loss of axons and secondary myelin in the long tracts of the spinal cord, possibly related to the impaired lipid metabolism of VLCFAs and the associated alterations (ie, oxidative damage). Similar lesions are encountered following inactivation of ABCD1 in mice (ABCD1(-)). A different and more aggressive phenotype is secondary to cerebral demyelination, very often accompanied by inflammatory changes in the white matter of the brain and associated with activation of T lymphocytes, CD1 presentation and increased levels of cytokines, gamma-interferon, interleukin (IL)-1alpha, IL-2 and IL-6, Granulocyte macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-alpha, chemokines and chemokine receptors.
Figures


References
-
- Asheuer M, Bieche I, Laurendeau I, Moser A, Hainque B, Vidaud M, Aubourg P (2005) Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X‐linked adrenoleukodystrophy. Hum Mol Genet 14:1293–1303. - PubMed
-
- Aubourg P (1996) X‐linked adrenoleukodystrophy. In: Handbook of Clinical Neurology, Vinken PJ, Bruyn GW, Moser HW (eds), pp. 447–483. Elsevier: Amsterdam.
-
- Aubourg P, Adamsbaum C, Lavallard‐Rousseau MC, Lemaitre A, Boureau F, Mayer M, Kalifa G (1992) Brain MRI and electrophysiologic abnormalities in preclinical and clinical adrenomyeloneuropathy. Neurology 42:85–91. - PubMed
-
- Aubourg P, Adamsbaum C, Lavallard‐Rousseau MC, Rocchiccioli F, Cartier N, Jambaque I et al (1993) A two‐year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. N Engl J Med 329:745–752. - PubMed
-
- Aubourg P, Blanche S, Jambaque I, Rocchiccioli F, Kalifa G, Naud‐Saudreau C et al (1990) Reversal of early neurologic and neuroradiologic manifestations of X‐linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med 322:1860–1806. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

