Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals
- PMID: 20626883
- PMCID: PMC2917405
- DOI: 10.1186/1617-9625-8-8
Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals
Abstract
Background: Nicotine replacement therapy (NRT) is the most common form of smoking cessation pharmacotherapy and has proven efficacy for the treatment of tobacco dependence. Although expectations of mild adverse effects have been observed to be independent predictors of reduced motivation to use NRT, adverse effects associated with NRT have not been precisely quantified.
Objective: A systematic review and meta-analysis aimed to identify all randomized clinical trials (RCTs) of NRT versus inert controls and all observational studies to determine the magnitude of reported adverse effects with NRT.
Methods: Searches of 10 electronic databases from inception to November 2009 were conducted. Study selection and data extraction were carried out independently in duplicate. RCTs were pooled using a random effects method with Odds Ratio [OR] as the effect measure, while proportions were pooled from observational studies. A meta-regression analysis was applied to examine whether the nicotine patch is associated with different adverse effects from those common to orally administered NRT.
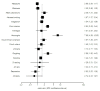
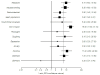
Results: Ninety-two RCTs involving 32,185 participants and 28 observational studies involving 145, 205 participants were identified. Pooled RCT evidence of varying NRT formulations found an increased risk of heart palpitations and chest pains (OR 2.06, 95% Confidence Interval [CI] 1.51-2.82, P < 0.001); nausea and vomiting (OR 1.67, 95% CI 1.37-2.04, P < 0.001); gastrointestinal complaints (OR 1.54, 95% CI, 1.25-1.89, P < 0.001); and insomnia (OR 1.42, 95% CI, 1.21-1.66, P < 0.001). Pooled evidence specific to the NRT patch found an increase in skin irritations (OR 2.80, 95% CO, 2.28-3.24, P < 0.001). Orally administered NRT was associated with mouth and throat soreness (OR 1.87, 95% CI, 1.36-2.57, P < 0.001); mouth ulcers (OR 1.49, 95% CI, 1.05-2.20, P < 0.001); hiccoughs (OR 7.68, 95% CI, 4.59-12.85, P < 0.001) and coughing (OR 2.89, 95% CI, 1.92-4.33, P < 0.001). There was no statistically significant increase in anxiety or depressive symptoms associated with NRT use. Non-comparative observational studies demonstrated the prevalence of these events in a broad population.
Conclusion: The use of NRT is associated with a variety of side effects. In addition to counseling and medical monitoring, clinicians should inform patients of potential side effects which are associated with the use of NRT for the treatment of tobacco dependence.
Figures
References
-
- Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. - PubMed
-
- NICE. Smoking cessation services in primary care, pharmacies, local authorities and workplaces, particularly for manual working groups, pregnant women and hard to reach communities. Public Health Guidance PH10. 2008. http://www.nice.org.uk/guidance/index.jsp?action=byID&o=11925
LinkOut - more resources
Full Text Sources
Medical