PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture
- PMID: 20626911
- PMCID: PMC2911397
- DOI: 10.1186/1477-3155-8-15
PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture
Abstract
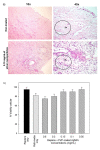
Background: Previous in vitro studies have demonstrated that polyvinylpyrrolidone coated silver nanoparticles (PVP-coated AgNPs) have antiviral activity against HIV-1 at non-cytotoxic concentrations. These particles also demonstrate broad spectrum virucidal activity by preventing the interaction of HIV-1 gp120 and cellular CD4, thereby inhibiting fusion or entry of the virus into the host cell. In this study, we evaluated the antiviral activity of PVP-coated AgNPs as a potential topical vaginal microbicide to prevent transmission of HIV-1 infection using human cervical culture, an in vitro model that simulates in vivo conditions.
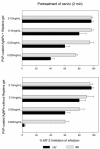
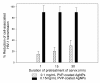
Results: When formulated into a non-spermicidal gel (Replens) at a concentration of 0.15 mg/mL, PVP-coated AgNPs prevented the transmission of cell-associated HIV-1 and cell-free HIV-1 isolates. Importantly, PVP-coated AgNPs were not toxic to the explant, even when the cervical tissues were exposed continuously to 0.15 mg/mL of PVP-coated AgNPs for 48 h. Only 1 min of PVP-coated AgNPs pretreatment to the explant was required to prevent transmission of HIV-1. Pre-treatment of the cervical explant with 0.15 mg/mL PVP-coated AgNPs for 20 min followed by extensive washing prevented the transmission of HIV-1 in this model for 48 h.
Conclusions: A formulation of PVP-coated AgNPs homogenized in Replens gel acts rapidly to inhibit HIV-1 transmission after 1 min and offers long-lasting protection of the cervical tissue from infection for 48 h, with no evidence of cytotoxicity observed in the explants.Based on this data, PVP-coated AgNPs are a promising microbicidal candidate for use in topical vaginal/cervical agents to prevent HIV-1 transmission, and further research is warranted.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

