The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in Netrin-mediated axon attraction and branching
- PMID: 20627077
- PMCID: PMC2974572
- DOI: 10.1016/j.devcel.2010.02.019
The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in Netrin-mediated axon attraction and branching
Abstract
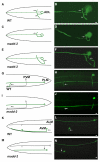
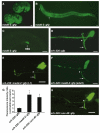
Neurons innervate multiple targets by sprouting axon branches from a primary axon shaft. We show here that the ventral guidance factor unc-6 (Netrin), its receptor unc-40 (DCC), and the gene madd-2 stimulate ventral axon branching in C. elegans chemosensory and mechanosensory neurons. madd-2 also promotes attractive axon guidance to UNC-6 and assists unc-6- and unc-40-dependent ventral recruitment of the actin regulator MIG-10 in nascent axons. MADD-2 is a tripartite motif protein related to MID-1, the causative gene for the human developmental disorder Opitz syndrome. MADD-2 and UNC-40 proteins preferentially localize to a ventral axon branch that requires their function; genetic results indicate that MADD-2 potentiates UNC-40 activity. Our results identify MADD-2 as an UNC-40 cofactor in axon attraction and branching, paralleling the role of UNC-5 in repulsion, and provide evidence that targeting of a guidance factor to specific axonal branches can confer differential responsiveness to guidance cues.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Acebes A, Ferrús A. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci. 2000;23:557–565. - PubMed
-
- Alexander M, Chan KK, Byrne AB, Selman G, Lee T, Ono J, Wong E, Puckrin R, Dixon SJ, Roy PJ. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136:911–922. - PubMed
-
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. - PubMed
-
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

