Antiviral immune responses in H5N1-infected human lung tissue and possible mechanisms underlying the hyperproduction of interferon-inducible protein IP-10
- PMID: 20627090
- PMCID: PMC2940995
- DOI: 10.1016/j.bbrc.2010.07.017
Antiviral immune responses in H5N1-infected human lung tissue and possible mechanisms underlying the hyperproduction of interferon-inducible protein IP-10
Abstract
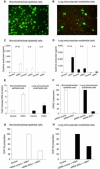
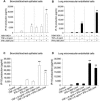
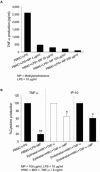
Information on the immune response against H5N1 within the lung is lacking. Here we describe the sustained antiviral immune responses, as indicated by the expression of MxA protein and IFN-alpha mRNA, in autopsy lung tissue from an H5N1-infected patient. H5N1 infection of primary bronchial/tracheal epithelial cells and lung microvascular endothelial cells induced IP-10, and also up-regulated the retinoic acid-inducible gene-I (RIG-I). Down-regulation of RIG-I gene expression decreased IP-10 response. Co-culturing of H5N1-infected pulmonary cells with TNF-alpha led to synergistically enhanced production of IP-10. In the absence of viral infection, TNF-alpha and IFN-alpha also synergistically enhanced IP-10 response. Methylprednisolone showed only a partial inhibitory effect on this chemokine response. Our findings strongly suggest that both the H5N1 virus and the locally produced antiviral cytokines; IFN-alpha and TNF-alpha may have an important role in inducing IP-10 hyperresponse, leading to inflammatory damage in infected lung.
Published by Elsevier Inc.
Figures

References
-
- Thitithanyanont A, Engering A, Ekchariyawat P, Wiboon-ut S, Limsalakpetch A, Yongvanitchit K, Kum-Arb U, Kanchongkittiphon W, Utaisincharoen P, Sirisinha S, Puthavathana P, Fukuda MM, Pichyangkul S. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol. 2007;179:5220–5227. - PubMed
-
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. - PMC - PubMed
-
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

