Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse
- PMID: 20627935
- PMCID: PMC2911065
- DOI: 10.2337/db09-1766
Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse
Abstract
Objective: Previous studies showed that genetic deletion or pharmacological blockade of the receptor for advanced glycation end products (RAGE) prevents the early structural changes in the glomerulus associated with diabetic nephropathy. To overcome limitations of mouse models that lack the progressive glomerulosclerosis observed in humans, we studied the contribution of RAGE to diabetic nephropathy in the OVE26 type 1 mouse, a model of progressive glomerulosclerosis and decline of renal function.
Research design and methods: We bred OVE26 mice with homozygous RAGE knockout (RKO) mice and examined structural changes associated with diabetic nephropathy and used inulin clearance studies and albumin:creatinine measurements to assess renal function. Transcriptional changes in the Tgf-beta1 and plasminogen activator inhibitor 1 gene products were measured to investigate mechanisms underlying accumulation of mesangial matrix in OVE26 mice.
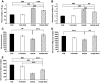
Results: Deletion of RAGE in OVE26 mice reduced nephromegaly, mesangial sclerosis, cast formation, glomerular basement membrane thickening, podocyte effacement, and albuminuria. The significant 29% reduction in glomerular filtration rate observed in OVE26 mice was completely prevented by deletion of RAGE. Increased transcription of the genes for plasminogen activator inhibitor 1, Tgf-beta1, Tgf-beta-induced, and alpha1-(IV) collagen observed in OVE26 renal cortex was significantly reduced in OVE26 RKO kidney cortex. ROCK1 activity was significantly lower in OVE26 RKO compared with OVE26 kidney cortex.
Conclusions: These data provide compelling evidence for critical roles for RAGE in the pathogenesis of diabetic nephropathy and suggest that strategies targeting RAGE in long-term diabetes may prevent loss of renal function.
Figures






References
-
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–1031 - PubMed
-
- Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D'Agati VD: Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol 2000;11:1656–1666 - PubMed
-
- Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM: RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 2003;162:1123–1137 - PMC - PubMed
-
- Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, Watanabe T, Shinohara H, Takeuchi M, Tsuneyama K, Hashimoto N, Asano M, Takasawa S, Okamoto H, Yamamoto H: RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin. Diabetes 2006;55:2510–2522 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

